Hide the solution

Hide the solution
| Electrical energy The salt bridge |
||
|
|
|
Look
at the cell on the left. At the anode more and more positive zinc ions
are being formed. While at the cathode more and more electrons are being
pumped into it. To remedy the situation we use a salt bridge. The salt bridge contains ions that complete the circuit by moving freely from the bridge to the half cells. These ions are carefully selected so as not to interfere with the reaction taking place. |
|
Click
to see a simple animation of how the salt bridge works.
|
||
|
After
viewing the salt bridge animation answer the questions below. Answer the
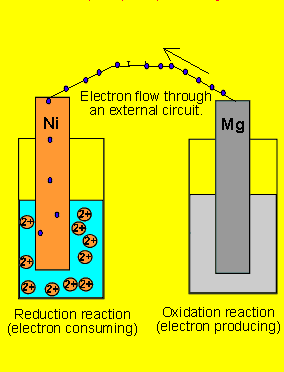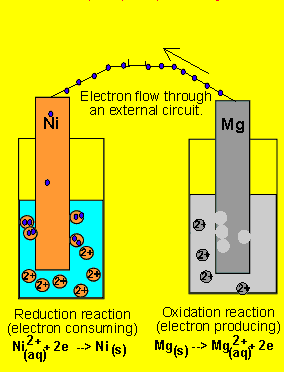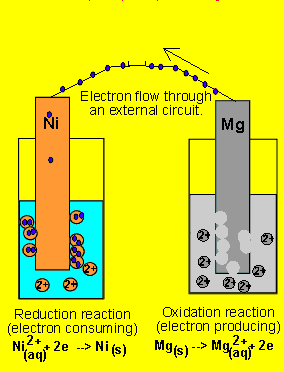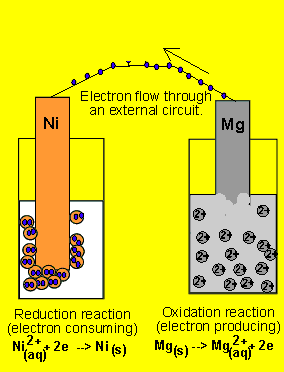
questions by assuming the animation is correct. 6) A galvanic cell(similar
to the one above) is set up by connecting the Ni2+(aq)
| Ni(s) and Mg2+(aq) | Mg(s half
cells via a wire and a salt bridge. The nickel electrode is noticed to
have increased in mass while the magnesium electrode has decreased. Draw
the galvanic cell indicating clearly the: 7) Draw a possible set up of
a galvanic cell using metal strips of zinc and copper and solutions of
copper sulfate and zinc nitrate. The salt bridge consists a filter paper
soaked in potassium nitrate. More exercises of setting up electrochemical cells from half cells. Continue with exercises to convert overall equations into an electrochemical cell |
||