The following pair of half cells are combined to form an electrochemical cell.
Fe2+(aq)/ Fe(s) and Cu2+(aq)/ Cu(s)
Draw a diagram of the electrochemical cell
Indicate the following
- direction of electron flow in the external circuit.
- direction of negative ions in the salt bridge.
- the anode
- the cathode
- the half equations at each electrode
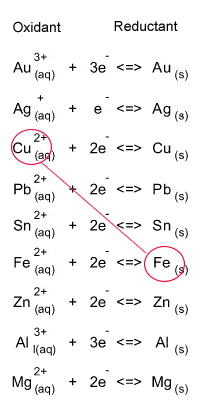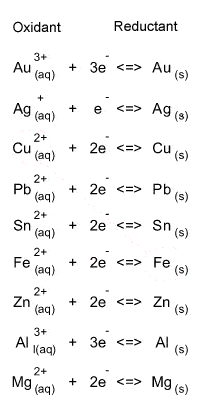
Step 1 Identify the oxidant and reductant from the electrochemical series, as shown on the right.
Oxidant = Fe
Reductant = Cu2+
Step 2 Write the two half reactions
Cu2+(aq) + 2e- → Cu(s) ----- reduction
Fe(s) → Fe2+(aq) + 2e- ----- oxidation
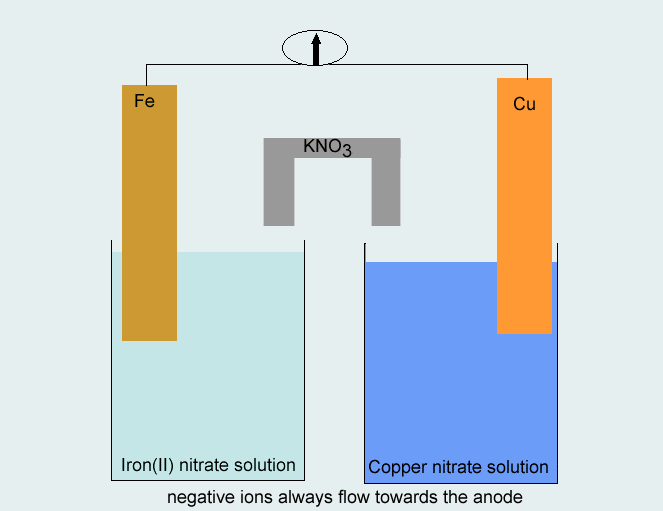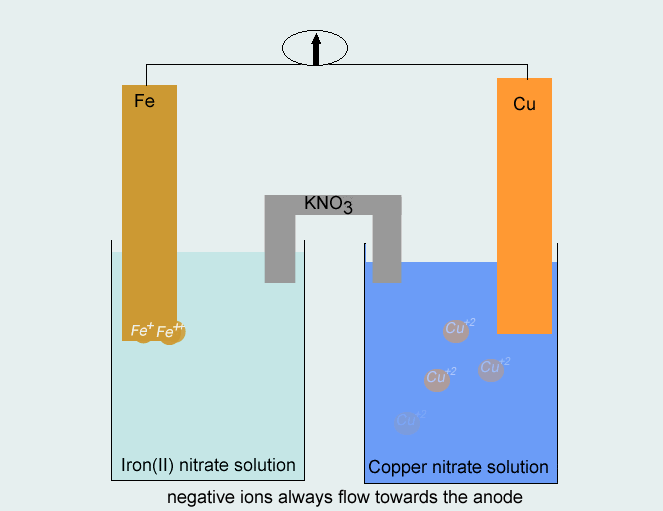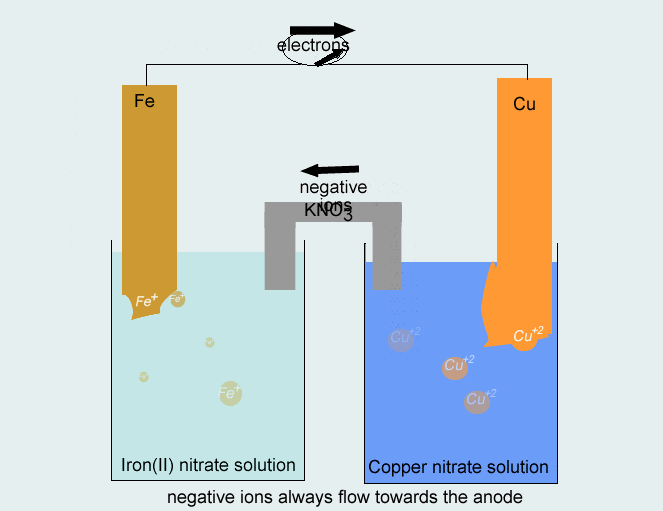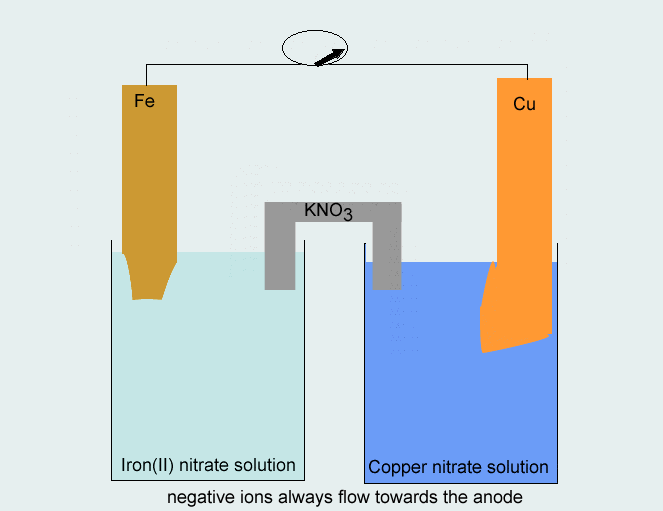
Step 3 Use the metals from each half equation as the electrodes in constructing the half cells. A salt solution containing the appropriate ions (Cu2+and Fe2+) should be used in each half cell. The direction of electron flow is from the half cell where oxidation takes place to the half cell where reduction takes place, whereas negative ions move in the opposite direction in the salt bridge.
The following pair of half cells are combined to form an electrochemical cell.
Ag+(aq)/ Ag(s) and Cu2+(aq)/ Cu(s)
Draw a diagram of the electrochemical cell
Indicate the following
- direction of electron flow in the external circuit.
- direction of negative ions in the salt bridge.
- the anode
- the cathode
- the half equations at each electrode
- what happens to the mass of the anode?
- what happens to the mass of the cathode?
Solution
Past exam question involving galvanic cells
Continue with some more exercises