Solution to Q6
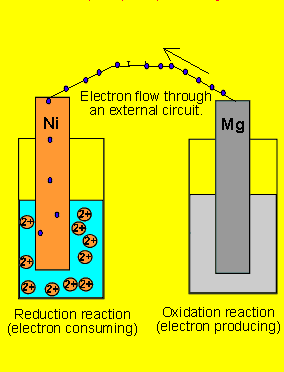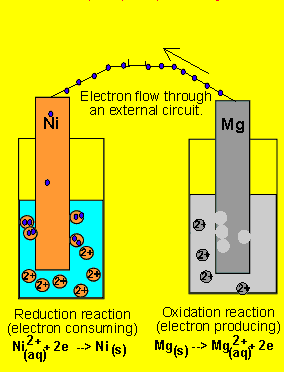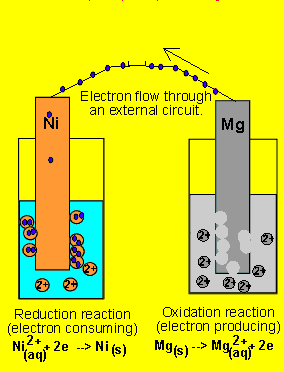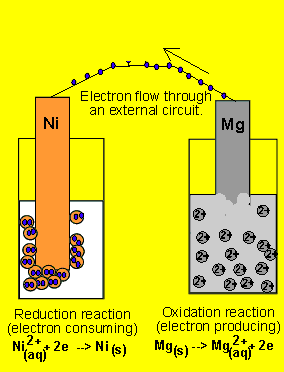
6) A galvanic cell(similar
to the one above) is set up by connecting the Ni2+(aq)/ Ni(s) and Mg2+(aq)/ Mg(s) half cells via
a wire and a salt bridge. The nickel electrode is noticed to have increased
in mass while the magnesium electrode has decreased. Draw the galvanic
cell indicating clearly the
- cathode and anode and the polarity.
- direction of electron movement
- the charge on the ions flowing in each half cell
- the reactions occuring in each half cell.
.