Solution to Q7
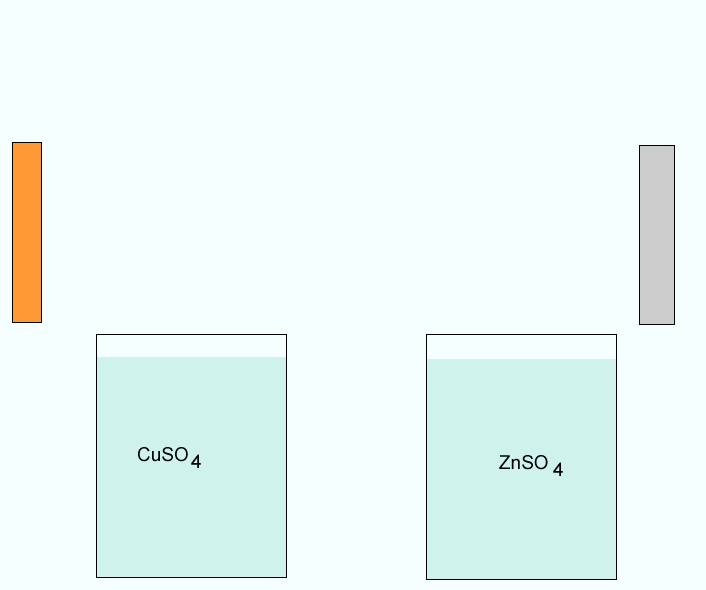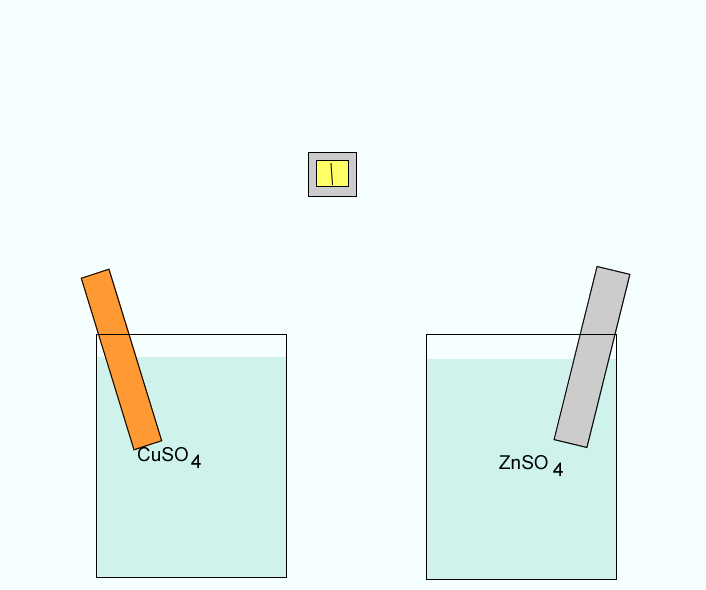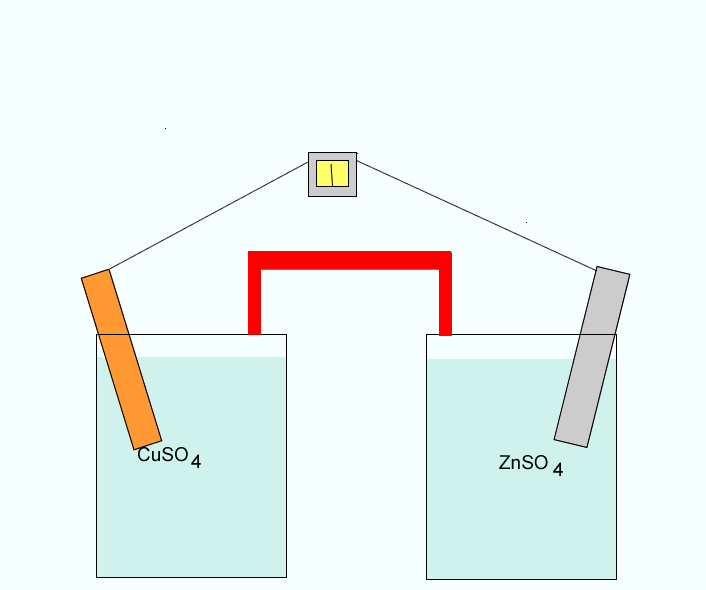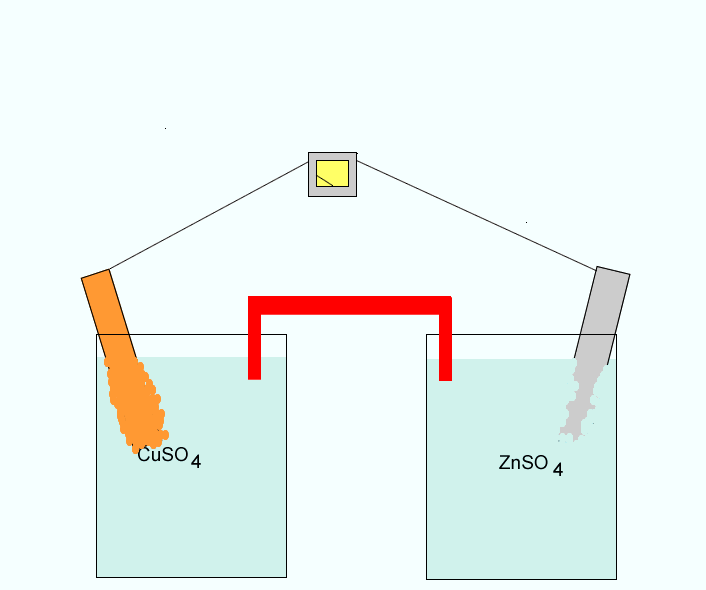
Click to see a closer view of the zinc electrode.
Click to see the anions coming from the salt bridge into the Zn/Zn2+ half cell.
Oxidation occurs at the anode
(-). The oxidation reaction can be written as Cu2+(aq) + 2e => Cu(s)
The copper electrode is the anode.
Reduction occurs at the cathode
(+). The reduction reaction can be written as
Zn(s) => Zn2+ + 2e
+ 2e