Electrical energy
Reactivity
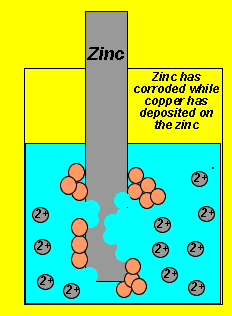
Why is it that copper ions take electrons from the zinc atoms while the zinc ions do not take electrons from the copper atoms? Both copper and zinc have the ability to give electrons the difference is that zinc has a greater ability than copper. We therefore say that zinc is a more reactive metal than copper.
Substances that take electrons from others are known as oxidants. While substances that give electrons to others are known as reductants. In this case the copper ion is the oxidant while the zinc metal atom is the reductant. In other words, copper ions take electrons while zinc atoms give electrons.

A small iron nail was placed in a solution of silver nitrate. Small crystals of silver metal formed on the surface of the iron nail as seen on the left.
1) Which is the more reactive metal out of silver and iron.
2) Identify the reductant and the oxidant.