Water molecules are very special.
Water (H2O) molecules are polar molecules. They have small charges on both ends that give them exceptional strength when it comes to attracting each other and other charged particles.
Click to see what creates these charges on the water molecule.
This force of attraction that exists between water molecules is known as hydrogen bonding.
View the video on the right to see a demonstration of the charged nature of water molecules as they get attracted to a charged object..
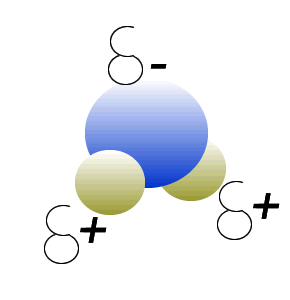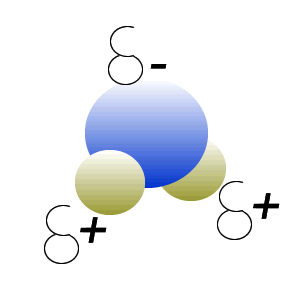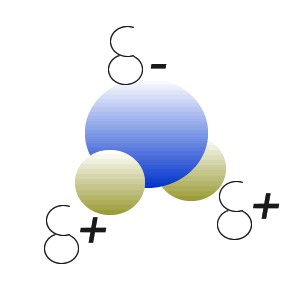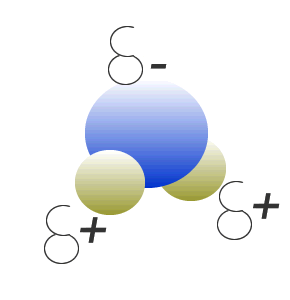
When cooled into a solid, water expands. This is why drink bottles left in the freezer will explode.
Ice is less dense than water and hence floats on the surface. Icebergs float, for this reason, and is why the Titanic came to its tragic end.
The reason why ice is less dense is outlined below.

What role does H-bonding play in the formation of ice that is less dense than the liquid state?
sourced from https://www.youtube.com/watch?v=KZ3szelrBLc at 7.57 2/05/21

Water molecules are held close together by relatively strong hydrogen bonding. At room temperature hydrogen bonds are broken and reformed repeatedly as shown on the right.
When water molecules are cooled down hydrogen bonding traps the molecules into a rigid three dimensional structure that aligns water molecules in such a way as to create huge gaps between the molecules. This makes ice less dense than water.
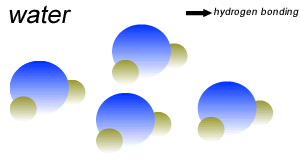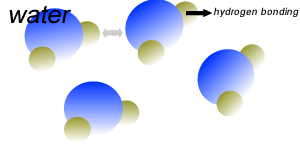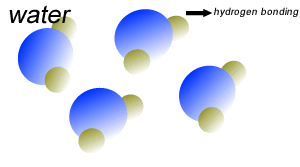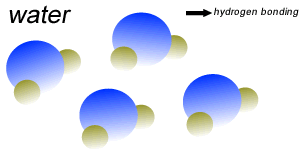
The small charges on the surface of the water molecule help make water an excellent solvent. These charges attract charged particles and help dissolve solids such as salt and sugar.
Click to see an animation (source unknown)

Hydrogen bonding is also responsible for the high surface tension of water. Hydrogen bonding holds the water molecules tightly at the surface and is the reason why small insects and even dense paper clips, as shown on the right, can remain on the surface. Even the so called Jesus lizard, which can walk on water, owes its special skill to hydrogen bonding and the high surface tension of water.
Try placing a paper clip on the surface of cold and hot water. Is there a difference?


Why does the surface tension of water reduce when it is heated?
Explain how water is able to dissolve solids such as salt or sugar?
Why does ice float on the surface of water?
Continue with capillary action and hydrogen bonding
Related topics, emulsifiers, surfactants activity, new theory of liquid water.