Food chemistry
Emulsions
In the preparation of our food we often have to mix oil and water. We know that the two liquids do not mix and the two separate out into different layers often giving the food a bad appearance and a gritty taste.
Salad dressings are often made from a mixture of oil and water. To stop the two liquids separating out into different layers special compounds are added called emulsifiers. Emulsifiers cause tiny droplets of one liquid, in this case oil, to be suspended in another liquid. Salad dressings are examples of suspensions where tiny oil droplets are suspended in water. Nearly all emulsions are composed of a polar phase, such as water and a non-polar phase, such as oil.
Shaking a bottle of oil and water, ofcourse, creates small bubbles of oil suspended in water. This occurs because energy is supplied to increase the surface energy of each liquid. When shaking has ceased the droplets of oil soon link together to form a layer of oil thus reducing the surface energy of both liquids.

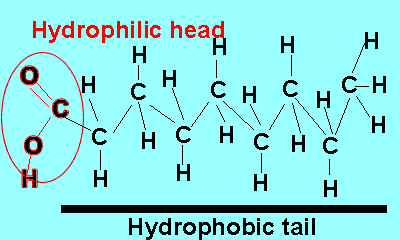
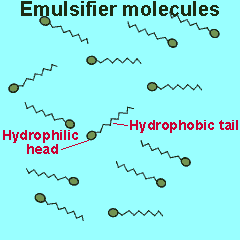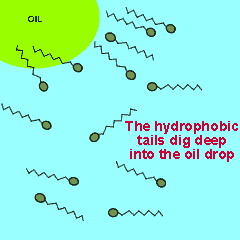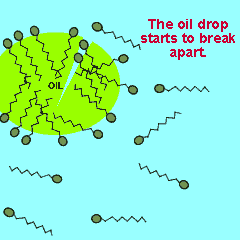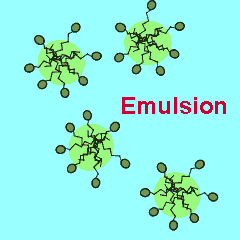
Emulsifiers are added to mixtures of oil and water. These compounds reduce the surface energy and therefore the tendency for liquids to separate. Emulsifiers belong to a class of compounds called surfactants. These compounds have a unique structure. The molecule is divided into two parts each with its own unique properties.
One part of the molecule, known as the head, is polar and hence hydrophilic (water loving) while the other is non-polar and hydrophobic (water repelling).
The polar end of the surfactant molecule forms ion-dipole or dipole-dipole bonds with the water. The non-polar tail can not participate in bonding with the water and orients itself away from the water, either in air or in oil droplets. As a result of this the surfactant molecules present in an oil water mixture orient themselves so that their tails are buried in the oil while their heads are protruding from the surface of the oil. This changes the properties of the surface of the oil and makes it interact more with the water molecules that surround it.
The animation on the right shows how the surfactant molecules orient themselves in an oil water mixture.