Hydrogen
bonding |
|||||||||||||
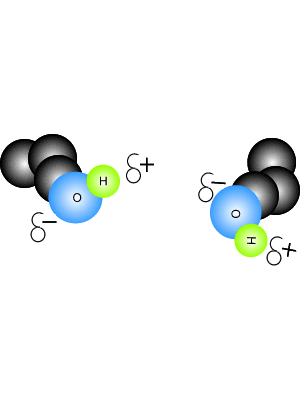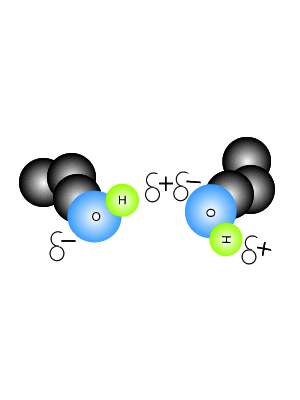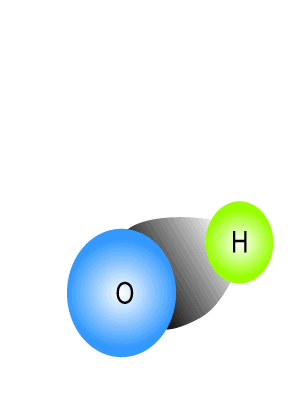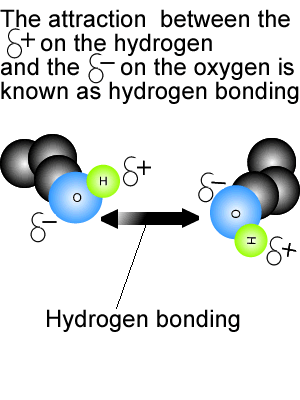
View the video on the right. It shows hydrogen bonding in water molecules. Answer the questions below. Hydrogen bonds are intermolecular bonds. That is, they occur between molecules or even between different parts of large molecules, such as proteins. Hydrogen bonding is a particularly strong form of dipole dipole bonding but not as strong as covalent or ionic bonding. It is an electrostatic force of attraction between molecules that have a hydrogen atom bonded to an oxygen, fluorine or nitrogen atom. - Hydrogen bonds are - Hydrogen bonds take place between molecules that have a - Explain how hydrogen bonding comes about.
|
sourced at https://www.youtube.com/watch?v=aZ8JxFwR_nY on 8.59pm 26/9/20 |
||||||||||||
The electronegativity
of some of the atoms are given below. The electronegativity of an element
is a measure of the relative attraction that the element has for electrons
in a bond.
It makes sense to assume that where there is great discrepancy in electronegativity between the two atoms in the bond the greater the charge on the dipoles will be. Consider hydrogen with an electronegativity of 2.1 and oxygen with an electronegativity of 3.5. Oxygen's greater attraction will mean that the electrons being shared will be pulled even closer to the oxygen atom and thus creating bigger charges (dipoles). When you compare the dipoles in water with those in hydrogen chloride(HCl) you would expect that the dipoles in HCl will be smaller due to the lesser discrepancy in electronegativity between hydrogen and chlorine.
. |
The animation
above shows the charges formed on the surface of a molecule by the uneven
sharing of electrons within chemical bonds. Notice how the electrons are
being pulled closer to the oxygen atom, hence creating a polar(charges
on both ends) molecule. |
||||||||||||
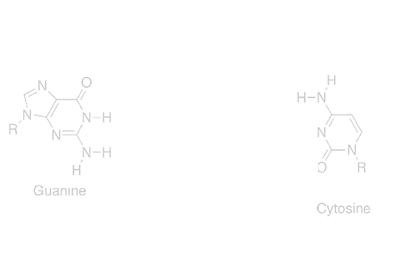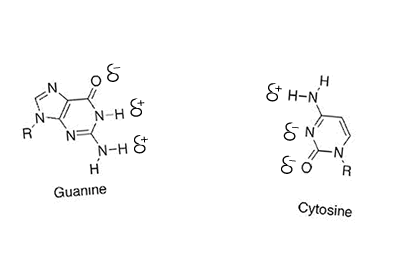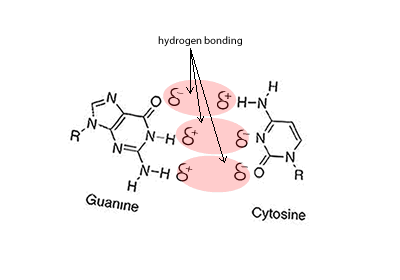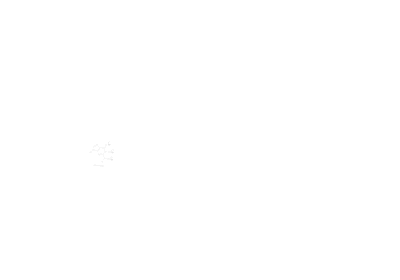
Hydrogen bonding can occur between a hydrogen bonded to either a nitrogen, oxygen or fluorine but also a nitrogen or oxygen bonded to any other atom. Look at the animation on the right between cystine and guanine. You will see that the oxygen and nitrogen atoms, even though they are not bonded to a hydrogen still carry a slight negative charge. Try these questions Will hydrogen bonding occur between Solution
|
|
||||||||||||
2) Formaldehyde and methane Solution |
|||||||||||||
3) Methanol and ammonia Solution |
|||||||||||||
4) Methyleneamino and formaldehyde Solution 5) Click to show an image of 5 amino acids linked together to form a small peptide. Hydrogen bonding will take place between what amino acids. Solution |
|||||||||||||
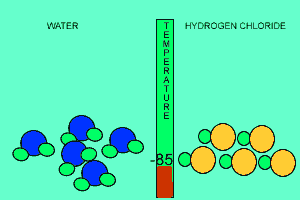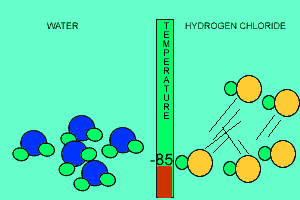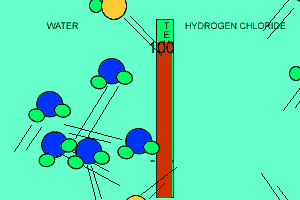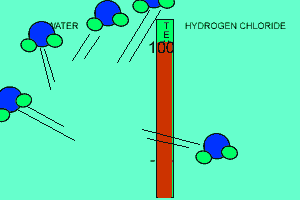
The animation above shows the relative strengths of the dipoles of hydrogen chloride and water. Clearly, water boils at 100 degrees Celsius while hydrogen chloride boils at -85 degrees Celsius. This tells us that the water molecules are held together more strongly than HCl molecules. This is due to the fact that dipole-dipole bonding is exceptionally strong in water. |
Water contains this rather powerful dipole-dipole attraction called hydrogen bonding. Hydrogen bonding gives water
very unique properties and is involved in a great number of other important
chemical functions such as: |
||||||||||||
| Continue with properties of water due to hydrogen bonding (junior science) | |||||||||||||