PVA to Gak
A study of hydrogen bonding.
We
can use the long polymers in PVA glue to make Gak. Gak is viscoelastic
material that can be squeezed and stretched.
You will need:
PVA glue (15 ml)
Distilled water (25 ml)
Talcum powder (5 ml)
Food dye (2 drops)
Borax solution 4% (5 ml)
Beaker
Teaspoon







As soon as the borax is placed into the PVA the mixture thickens. Something is holding the long PVA polymers together. But at the same time they are not permanently held in place as the putty is allowed to run and form new shapes. The chemistry behind this is very simple. Below is a diagram of how this occurs. The borate ion form temporary bonds with the polymers. These bonds are formed by hydrogen bonding and can be easily broken and reformed. These allows the polymers to slide past each other while still being kept together.
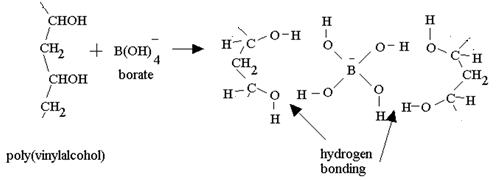
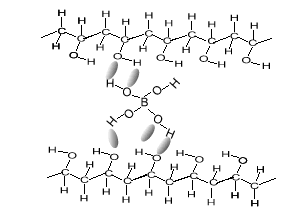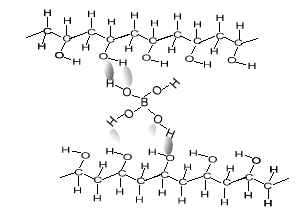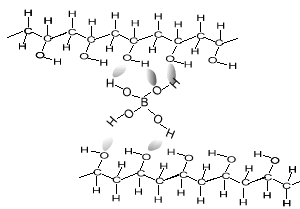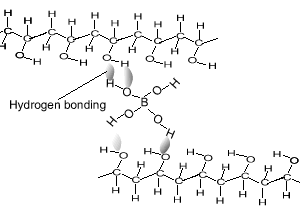
Consider the animation above.
Between what two atoms does
hydrogen bonding occur?
Are hydrogen bonds permanent?
Is hydrogen bonding as strong as the bonds that keep the atoms together
in the polymer?
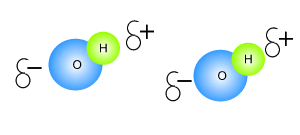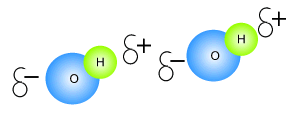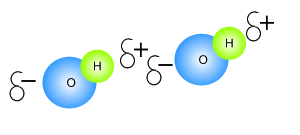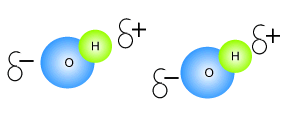
Click to see an explanation of how hydrogen bonding comes about and answer the following questions.
The atom that attracts the
electrons the strongest has
The size of the charge on the atoms depends on the