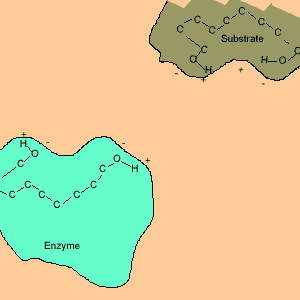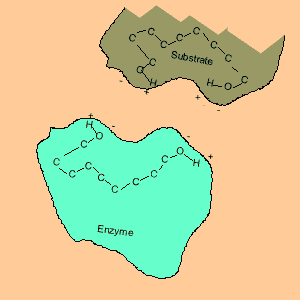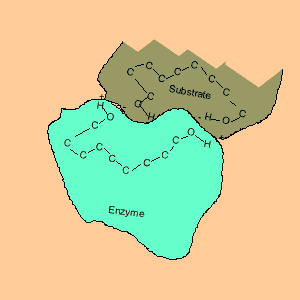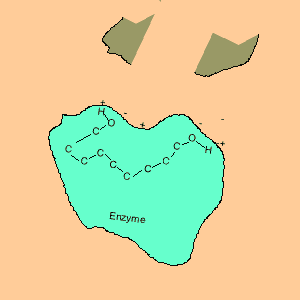
Animation of an enzyme acting as a catalyst. Notice how the dipoles are matched on the enzyme and reactant. This alignment gives the enzyme specificity for the reactant. Look carefully at how the dipoles, in this case hydrogen bonding, come about (bonding of hydrogen to oxygen).
Many biological processes rely heavily on hydrogen bonding. The specific nature with which the DNA bases combine and the recognition of antibodies for antigens all rely on hydrogen bonding.
Enzymes are chemical catalysts in our bodies. Their action starts as soon as food enters our mouth.They control reactions that are vital and life sustaining. They depend on recognising and bonding to specific reactants that are needed to produce vital products. The recognition and bonding is completed by hydrogen bonding. The enzyme has a complementary set of dipoles on its surface that match exactly those on the reactant. No other reactant can bond to this enzyme due to the specific placement of the dipoles.