Surface tension activity.
Fireworks in milk
- watch-glass;
- detergent:
- fullcream milk;
- eye dropper;
- food dye.



Now place a drop of detergent in the middle and watch.
Click to see a 800 kb video.
Complete the investigation worksheet

After viewing the video you will notice that the water is pulled to the edges away from the drop of detergent. The detergent is a surfactant and lowers the surface tension of water. That is, it destroys the attraction between the water molecules. Water molecules at the surface are therefore pulled away from the area of low surface tension, as shown in the animation on the right.
Click to see another animation.
.
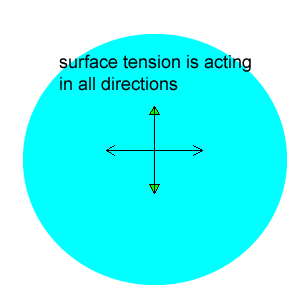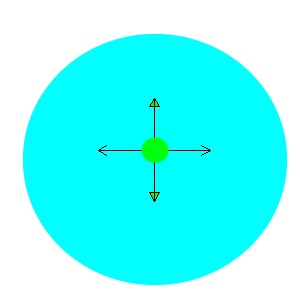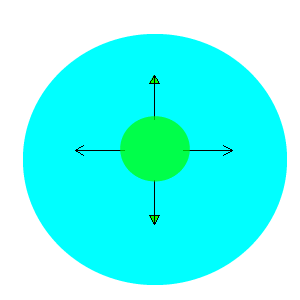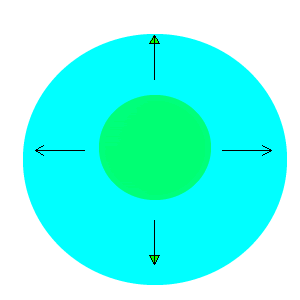

Why do the drops of water soluble dye not spread out in the milk?
Would this work just as well with skim milk? Try it.
Does warm milk make any difference to the speed of colour flow in the milk? Try it.
Does the flow of colour in the milk slow down if you seal the watch-glass with Glad-Wrap? Try it.
Two water droplets on a piece of cloth are shown on the right. Which water droplet contains a surfactant. Explain
Surfactants make water wetter. That is, the water is able to make clothing wetter. Explain
Does water with detergent added boil at a lower temperature?