A special method exists in
naming organic compounds. When faced with having to name an organic molecule
we follow the steps below.
Step 1 We identify the longest
carbon chain in the molecule. The example on the right has butane as the
longest carbon chain.
Step 2 We number each carbon atom in the chain so that the substituents
are linked to the lowest numbered carbon atoms.
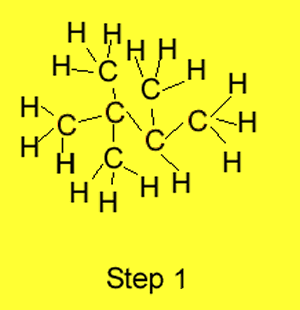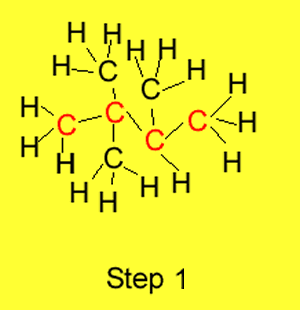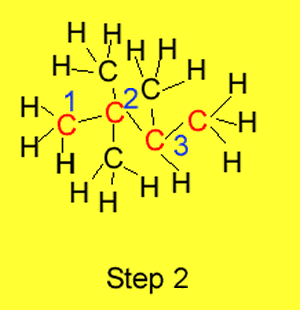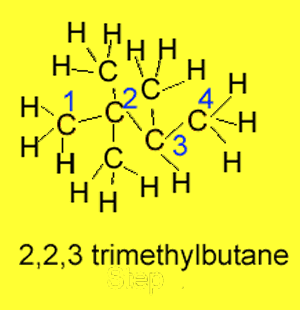
Step 3 Name, as prefixes, the
alkyl and or halide groups. In the example on the right we have 3 methyl
groups coming off the butane.
Step 4 If the same substituent
group appears more than once prefixes such as "di"
for 2 or "tri" for 3 are used. In the example
on the right we have
2,2,3-trimethylbutane.
Step 5 If more than one substituent
group appears it is placed in the name in alphabetical order. For example
if butane had a chlorine(chloro) atom on carbon number 1, a methyl group
and fluorine(fluoro) atom on carbon number 2 then the name would be written
as 1-chloro-2-fluoro-2-methylbutane.
Rules for punctuation:
1. Commas are used to separate numbers. For example, the numbers 2 4 5 become 2,4,5
2. Hyphens(-) are to separate a number from word. For example 2,3 dichloropropane becomes 2,3-dichloropropane.3. No space between words. For example trichloro butane becomes trichlorobutane.
This generates a one word name for each compound.