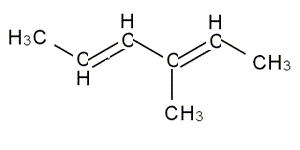
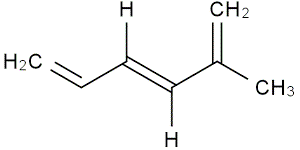
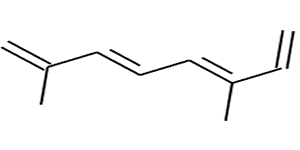
Molecules with more than one
C=C double bond.
When dealing with molecules which have more than one double carbon to carbon bond follow the following steps.
1) Determine the longest chain of carbon atoms that includes the double bonds.
2) Name the carbon atoms so that the double bonds are on the lowest numbered carbons.
3) Indicate the position of each C=C double bond by using the number of the first carbon of each double bond and use the suffix -diene (for 2 double bonds), -triene (for 3 double bonds), -tetraene (for 4 double bonds) and so on.
Let's see an example.
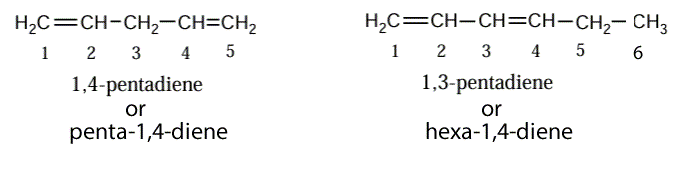
In the presence of multiple double bonds, however, the following steps are taken:
-
number of double bonds is indicated by the appropriate suffix such as -diene or -triene. Numbers before the suffix give the carbon number that is relevant to the location of each carbon to carbon double bond on the backbone or parent carbon chain.
-
only -ne is removed from the parent alkane chain name leaving an "a" in the name.
Let's see an example
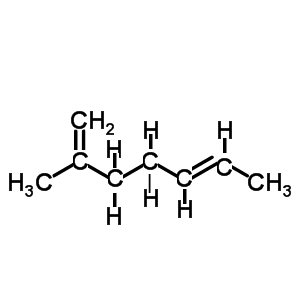
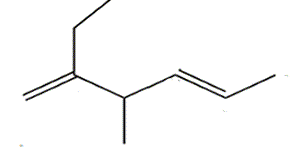
Name the diene shown on the left.
- select the longest chain that includes both C=C bonds.
It is a hepta-diene
- number the carbons so that the C=C are on the lowest possible numbered carbons.
hepta-1,5-diene
- identify the substituent groups and the carbon number to which they are attached and add it to the name as a prefix.
2-methylhepta-1,5-diene