So the common names of the first four aldehydes become:
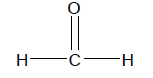
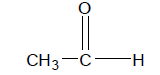
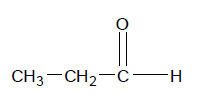
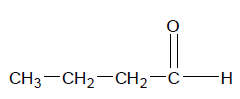
Now this is all pretty simple, but what if there are side chains off the main aldehyde chain. For example, take the molecule pictured on the right.
Step 1 The aldehyde forms the root name
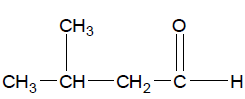
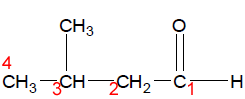
Step 3 Name all the substituents in alphabetical order.
3-methylbutanal
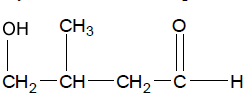
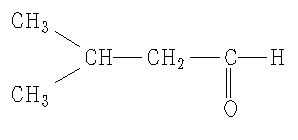
Now lets look at the steps to derive a IUPAC name.
Step 1) Identify the longest carbon chain that contains the carbonyl group and number the carbons so that the carbonyl group is on the lowest carbon.
Step 2) Identify the various substituent groups
Step 3) Place the substituent groups in the name in alphabetical order and use position numbers to locate the substituent groups and the carbonyl group.
Step 4)
Change the end e to a one.
Step 5) Write the name with the locator number, if ambiguity exists, for the carbonyl group.
In this case the name is propanone, as no ambiguity exists.
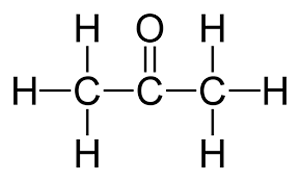
Lets take the molecule shown on the right as an example.
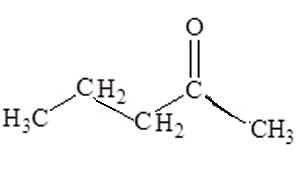
Step 1 The longest carbon chain is pentane
Step 2
No substituent groups
Step 3
and 4 The carbonyl group is found at carbon number 2 .
Hence the name is 2-pentanone or pentan-2-one. Both names are correct and commonly used interchangeably.
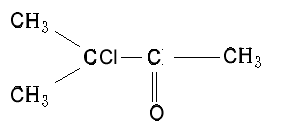
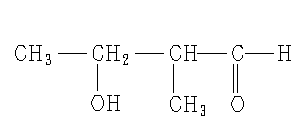
What is the IUPAC name of this molecule?
Solution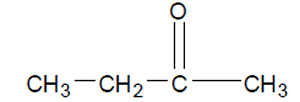
What is the IUPAC name of this molecule?
Solution
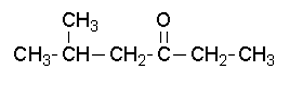
What is the IUPAC name of this molecule?
Solution
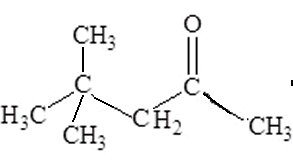
Name the molecule on the right
Solution