Alcohols
Name |
Formula |
Structural
formula |
Methanol |
CH3OH |
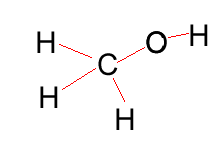 |
Ethanol |
CH3CH2OH |
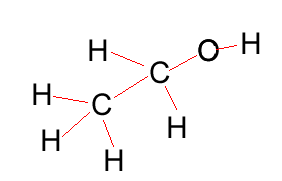 |
Propanol |
CH3CH2CH2OH |
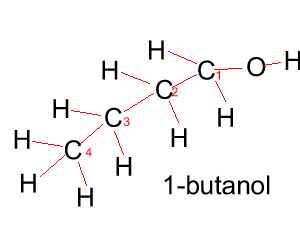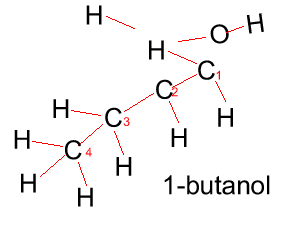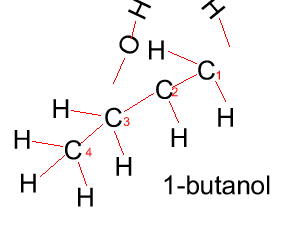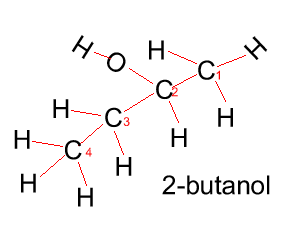
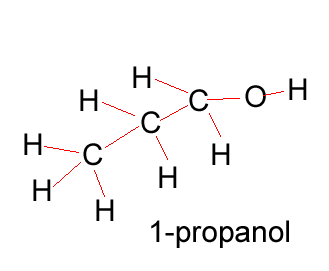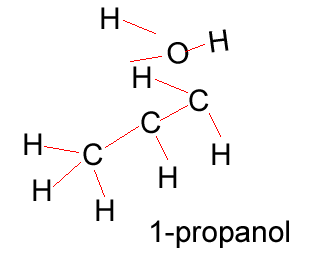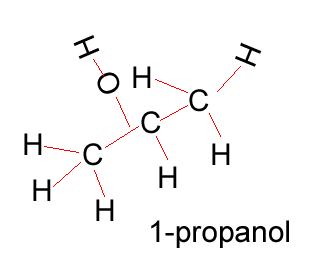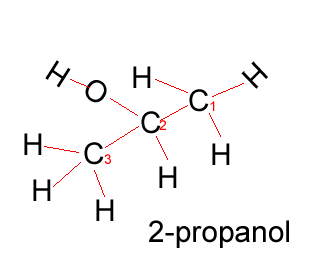 Propanol has two forms as does butanol, shown below. 1-propanol and 2-propanol are isomers. That is, they have the same molecular formula but a different structural formula. |
Butanol |
CH3CH2CH2CH2OH |
 |
| What about if an alcohol has two hydroxyl groups? Well we count the longest carbon chain that contains the two hydroxyl groups, keep the "e" at the end of parent chain, number the carbons so that they are the lowest possible numbers and use the suffix diol. Lets see some examples | ||
| Ethane-1,2-diol | 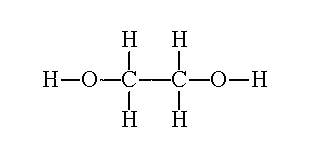 |
|
| Pentane-1,2-diol | 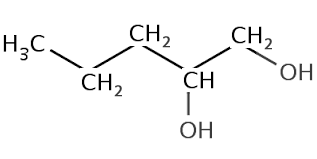 |
|
Keep in mind that it is Ok to write 4-butanol as butan-4-ol. It is up to you. 3-hexanol, for example, can be written as hexan-3-ol.
Why do we not
use the names 4-butanol or 3-butanol?
Solution
How
many different straight carbon chain isomers of pentanol can you identify? Draw their structural
formula.
Solution
Continue with naming alcohols primary as well as secondary and tertiary alcohols