Alcohols contain the hydroxy group (R- OH) and take the suffix "ol" with an infix numerical bonding position, for example:
- propan-2-ol
CH3CHOHCH3
- butan-2-ol
CH3CHOHCH2CH3
- hexan-3-ol
CH3CH2CHOHCH2CH2CH3
- pentan-1-ol
CH3CH2CH2CH2CH2OH
Step 2 Number the carbons so that the -OH group is on the lowest carbon.
Step 3 Treat the side branches as you would normally.
Step 4 Use suffixes -diol, -triol, -tetraol when multiple hydroxy groups are present and keep the "e". See examples below.
a) ethane-1,2-diol
b) butane-1,2,3-triol
c) propane-2,2-diol
c) ethylene glycol (CH2OHCH2OH) is used as an automotive antifreeze agent. It can also be called?
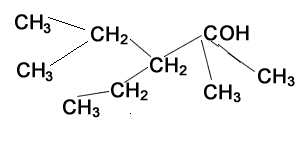
Consider the structure shown on the right.
Step 1) Identify the longest carbon chain (parent chain) and number the carbons
Step 2) Identify the substituent chains branching off the parent chain
Step 3) Write the name
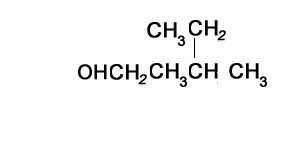
The compound shown on the left represents a secondary alcohol. That is the carbon with the -OH group is bonded to two other carbons.
Solution
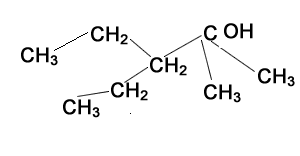
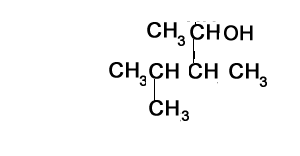
The compound shown on the left represents a tertiary alcohol. That is the carbon with the -OH group is bonded to three other carbons.
Solution