Hide solution
Because atoms can rotate about single covalent bonds 1-butanol is the same molecule as 4-butanol and 2-butanol is the same molecule as 3-butanol.
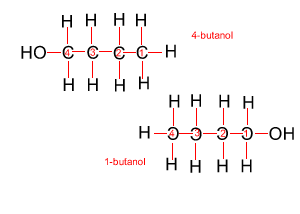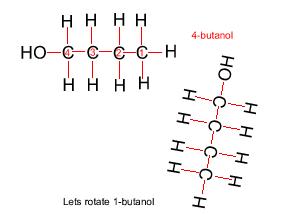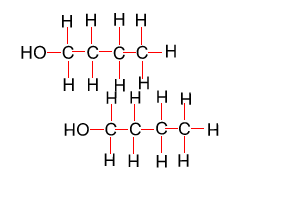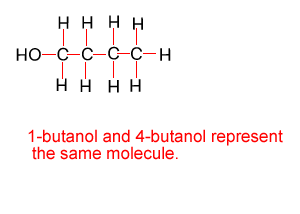
Hide solution
Because atoms can rotate about single covalent bonds 1-butanol is the same molecule as 4-butanol and 2-butanol is the same molecule as 3-butanol.

| Alcohols and their reactions with oxidants |
Primary alcohols can be oxidised to form aldehydes or carboxylic acids depending on the reaction conditions. In the formation of a carboxylic acid, the alcohol is first oxidised to an aldehyde which is then oxidised further to the acid. This occurs only if there is an excess of oxidising agent placed in with the primary alcohol. If the reaction mixture is controlled, however, so that the aldehyde is removed as soon as it is formed and the oxidising agent is the limiting reactant then the product is predominantly an aldehyde. Only when excess oxidising agent is used will the aldehyde further oxidise into a carboxylic acid. |
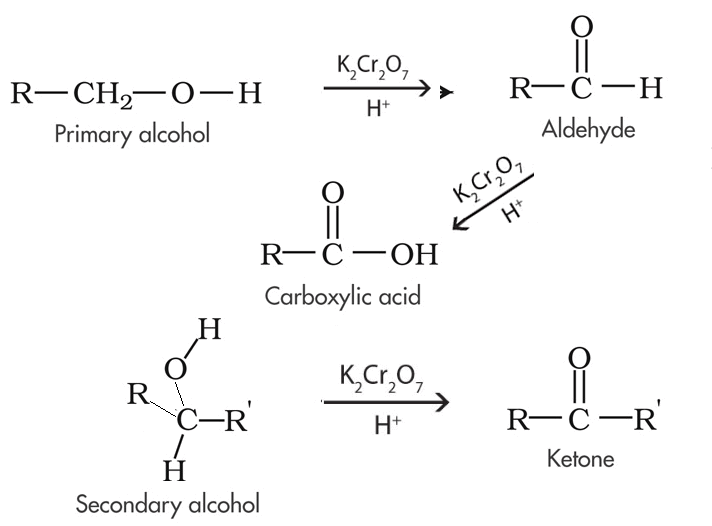 Secondary alcohols are simply oxidised to ketones in the presence of an oxidant. |
1) Propan-1-ol was placed in a reaction chamber with excess potassium dichromate(VI) solution acidified with dilute sulphuric acid. What is the name of the product formed?
|
| 2) Excess butan-2-ol was placed in a reaction vessel with a potassium dichromate(VI) solution acidified with dilute sulphuric acid. What is the name of the product formed? |
| 3) Ethanol is placed in a reaction vessel with potassium dichromate(VI) solution acidified with dilute sulphuric acid. There is only one product formed. This product reacts with Na2CO3 to produce carbon dioxide. Which comment is true? |
5) A compound was placed in a reaction vessel containing excess potassium dichromate(VI) solution acidified with dilute sulphuric acid. The product of this oxidation reaction was analysed and found to contain 62.1% Carbon,10.3% hydrogen and 27.6% oxygen by mass. If its molar mass is 58.0 g/mol what is the original compound that reacted? |
| 6) Label the compounds A, B and C on the diagram below. |
|
| 7) A secondary alcohol was placed in an acidified solution containing a dilute acid. The compound 2-pentanone or methyl propyl ketone was formed. What was the secondary alcohol? |
| Exercise involving HNMR |