Enzymes
Enzymes are proteins that act as catalysts:
- they participate in the reaction, but are not used up or altered during the reaction.
- do not affect the overall yield of product, only the rate at which the reaction proceeds, between 108 and 1010 faster than normal rates in absence of an enzyme.
- specific for an individual reaction.
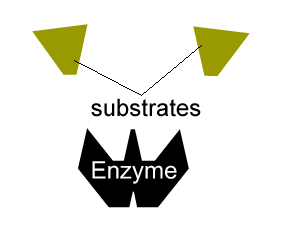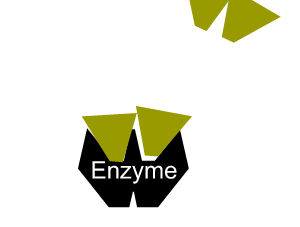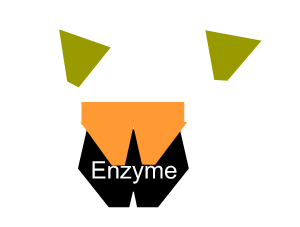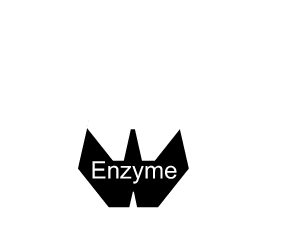
- reduce the activation energy through an alternative reaction pathway that brings the substrates into fixed positions on the surface of the enzyme to form a substrate-enzyme complex.
- they are specific to only one enantiomer (optical isomers of chiral molecules) .
All enzymes have a region on their surface where reactants attach, called the active site.
Enzymes work best within a narrow pH and temperature range. Heating the enzyme or altering the pH of the enzyme's environment can alter the tertiary structure of the protein and hence influence the shape of the active site and render the enzyme inactive. The enzyme is said to be denatured. Denaturing occurs when the protein is forced to unravel to a certain degree and thus lose its unique shape.

In the absence of enzymes most reactions proceed very slowly indeed. As shown on the right, in the absence of a catalyst the bonding of glucose molecules to form a branched polymer, glycogen, proceeds at a very slow rate. However the rate is increased in the presence of an organic catalyst (enzyme).
Enzymes lower the activation energy of a reaction.
Many enzymes cannot function on their own. A coenzyme is often needed to activate an enzyme. Coenzymes are usually small molecules significantly smaller than the enzyme they bind to and activate. Some of these coenzymes are derived from vitamins and are required in very small doses in our diet, this is why vitamins are sometimes referred to as micronutrients.
Coenzymes accept electrons or small groups of molecules and unlike enzymes are altered during the reaction. Coenzymes can be restored to their original state in subsequent reactions where the electrons or small molecules that they have have accepted are removed.
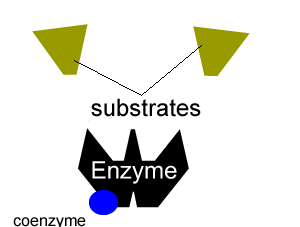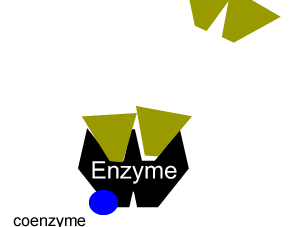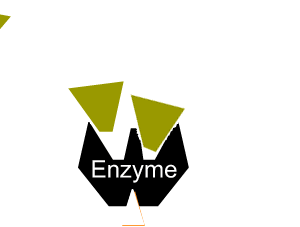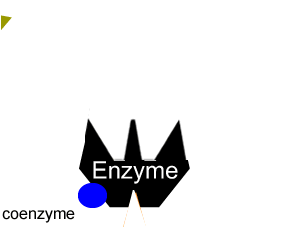
Enzymes can be inhibited in a number of ways that fall into two categories, reversible and irreversible.
Non-competitive inhibition involves the irreversible change to the shape of the active site.
In reversible inhibition a competitive equilibrium is setup with an inhibiting molecule which has a much greater Kc value than the reaction catalysed by the enzyme. As a result, the inhibiting molecule binds to the active site rather than the substrate and prevents the preferred reaction from taking place. This is reversible inhibition as the preferred reaction can be encouraged to proceed by increasing the concentration of substrate or reducing the concentration of the inhibiting molecule.