Optical isomers

Consider the two molecules shown on the right, one is a mirror image of the other. They look as though they are the same molecule, but as shown, no matter how we twist or turn these two molecules, we cannot get them to overlap each other. The two molecules are called enantiomers, and exhibit a form of isomerism known as optical isomerism.
Optical isomerism is another form of stereoisomerism, where the atoms making up the isomers are joined up in the same order, but have a completely different spatial arrangement.
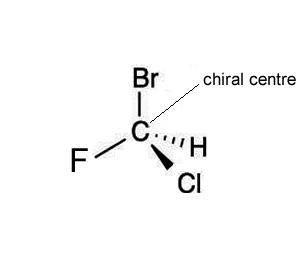
Consider the molecules CHClFBr shown on the right.
Click to rotate the molecule.
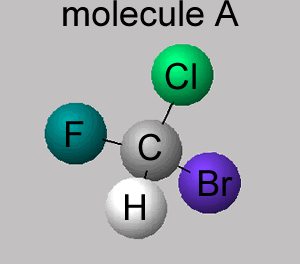
The molecule shown on the right is also CHClFBr, however, it is different, it has a different spatial arrangement of atoms around the carbon atom. Both molecules have the same group of atoms attached to each other and yet still manage to be different.
Comparing molecules A and B we can see that the fluorine and hydrogen atoms are in different locations, rotating the molecules to try and get them to align is impossible.
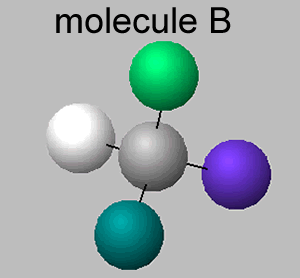
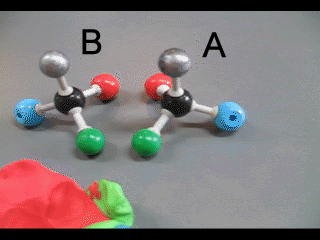
Enantiomers have identical physical properties. Chemical properties, however, depend on the environment in which the molecules interact. If the interaction is in an achiral environment then the chemical properties of both enantiomers are the same. If the interaction takes place in a chiral environment, that can distinguish between the two enantiomers, then the enantiomers will exhibit different chemical properties. Let's take the example of an achiral environment represented by a biological model of a protein receptor binding to a stimulus molecule.
Consider the two molecules "A" and "B" shown on the right. They are structurally similar but differ in the spatial arrangement of atoms around central carbon atoms.
Click
Molecule "A" fits nicely into the protein, represented by the plasticine, however, molecule "B" does not form a perfect fit no matter how we rotate the molecule around the two central carbon atoms. Molecule "A" will initiate a response as it binds perfectly but molecule "B" will not.
This demonstration also shows how enzymes, which are really protein catalysts, can distinguish between optical isomers.
In biological systems the spatial arrangement of atoms around a central carbon is critical to the chemical behaviour of the molecule within its biological environment. Such subtle differences amongst isomers provides for a countless number of possibilities when applied to living organisms.
Take the two enantiomers (optical isomers) shown on the right. Although they look identical, they bind differently to proteins in our noses, known as receptors, to produce smells of lemons or oranges.
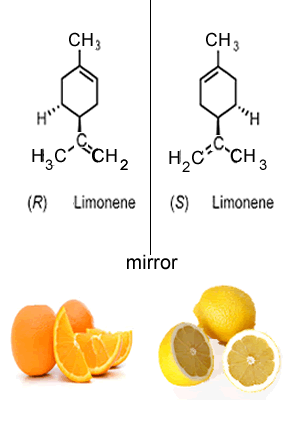
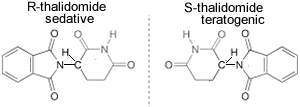
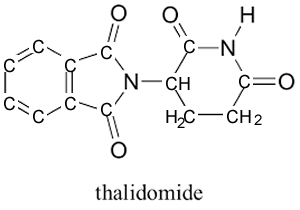
A famous case of the optical isomers of a compound having two opposite and extreme consequences on the body is that of thalidomide, the two optical isomers are shown on the right. Thalidomide was sold in the 1960's to pregnant women to combat nausea. (R) thalidomide helped against nausea, while (S) thalidomide caused fetal damage (teratogenic). During this period the drug was only available as a mixture of both isomers. This tragic episode was totally avoidable had each isomer been tested for its physiological effects prior to been commercialised. As a result of this case new procedures were adopted where all isomers of a given compound are now tested as separate drugs.
Lets look at two of the isomers of glucose, namely, the L-glucose and the D-glucose in their straight chain structure. The two isomers differ in the positioning of the hydroxy group on carbon number 5. Small difference indeed, however, while D-glucose is a product of photosynthesis, L-Glucose does not occur naturally and is synthesised artificially in the laboratory. Both the L-glucose and D-glucose isomers are indistinguishable in taste and yet L-glucose cannot be used by living organisms as an energy source because it cannot interact with an essential enzyme, hexokinase, in the glycolysis pathway. L-glucose can be used as an artificial sweetener but the cost of manufacture prohibits widespread use.
Lets take a close look at two other isomers of glucose, the alpha-D-glucose and
beta-D-glucose.
The alpha and beta isomers differ by the position of an hydroxy group on carbon number 1. In the alpha the hydroxy group is below the plane of the molecule where as in the beta isomer the hydroxy group is above the plane of the molecule. When alpha-D-glucose is polymerised in a condensation reaction two important polymers, polysaccharides, are formed known as starch and glycogen. These two polysaccharides are energy stores in organisms, starch is an energy store for plants and glycogen is an energy store for animals. Starch is easily digested by enzymes found in the digestive tract of animals. When beta-D-glucose is polymerised another unusual polysaccharide is formed, known as cellulose. Cellulose can not be digested by animals as they do not have enzymes capable of breaking down this polymer. Cellulose is a tough substance found in the cell wall of plant cells and other organisms and is predominantly a substance used for structural strength.
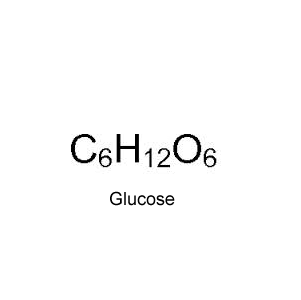
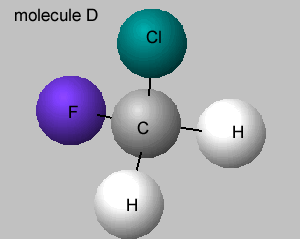
There is no symmetry around the central carbon if the four groups of atoms attached are different. If two groups are the same around the central carbon atom, then a line of symmetry exists, as shown on the right.
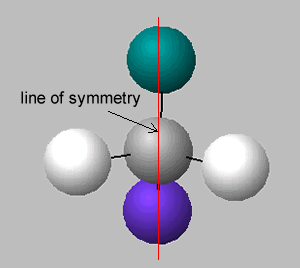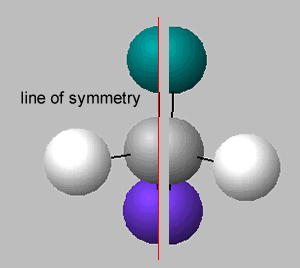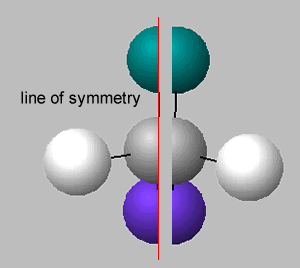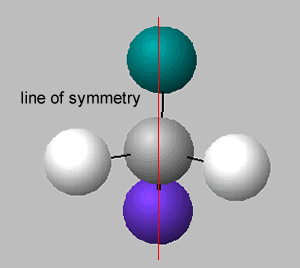
A molecule which has no line of symmetry is described as chiral. The carbon atom with four different groups attached, that causes this lack of symmetry, is known as a chiral centre. Complex molecules can have more than one chiral centre.
With few exceptions, molecules with one or an odd number of chiral centres are chiral molecules. Molecules that have an even number of chiral centres can sometimes be achiral, however, this course will deal with simple cases so you can assume that molecules with chiral centres are chiral.
if a molecule has a line of symmetry then it is not chiral but achiral.
Only chiral molecules have optical isomers.
How to identify chiral centres in molecules.
Basically you need to look for carbons that have four different sets of atoms attached.
The number of optical isomers is given by the formula
Number = 2y where y is the number of chiral centres in the molecule.
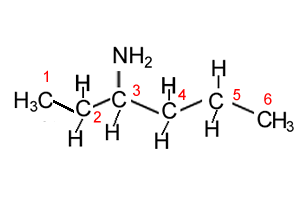
Lets see how we recognise chiral centres. Lets consider the molecule shown on the right. Is it a chiral molecule? In other words can we find a chiral centre?
For each carbon determine whether it is a chiral centre or not.
- carbon 1 - has three hydrogens atoms attached. Clearly it has a line of symmetry so is not a chiral centre.
- carbon 2 - has two hydrogens attached. Clearly it is also not a chiral centre due to align of symmetry.
- carbon
3 - has four different groups of atoms attached. It has a hydrogen atom, an amine group , a CH3CH2 and an CH2CH2CH3 group. So this is a chiral centre.
- carbon 4 - has two hydrogens so this has a line of symmetry so can not be a chiral centre.
- carbon 5 - is the same as carbon 4
- carbon 6 - is the same as carbon 1.
This molecule has one chiral centre at carbon 3.
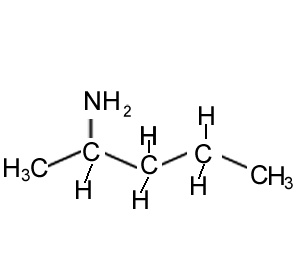
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible.
Solution
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible.
Solution
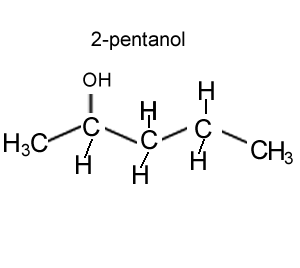
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible.
Solution
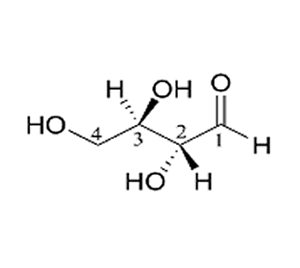
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible.
Solution
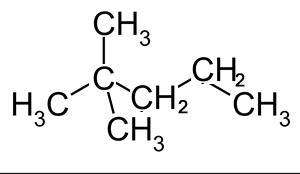
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
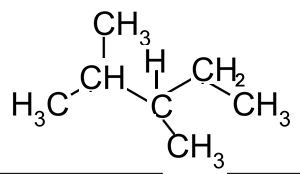
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
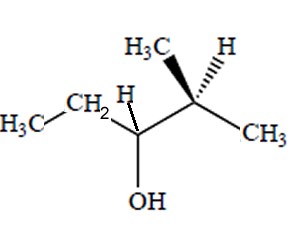
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
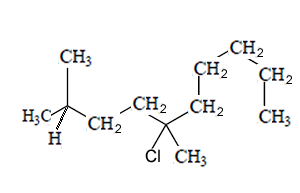
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
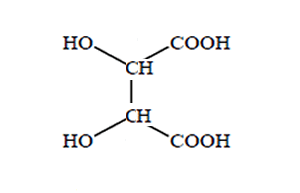
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
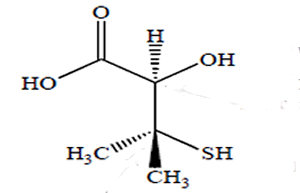
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
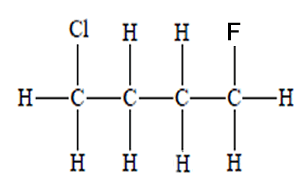
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
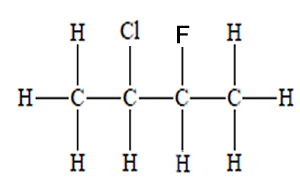
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
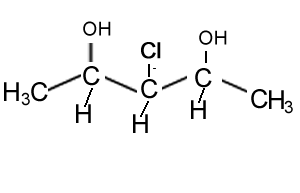
How many chiral centres are present in this molecule.
Identify the chiral centre
How many optical isomers are possible
Solution
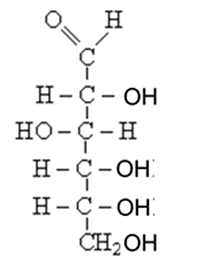
Draw the structural formula of the optical isomer with the molecular formula shown on the right. Show the chiral carbon atom.
Solution
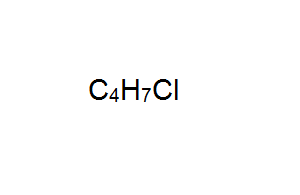
Although aromatic structures are not part of this unit follow the steps outlined above to identify the location and number of chiral carbon atoms in thalidomide.
Solution
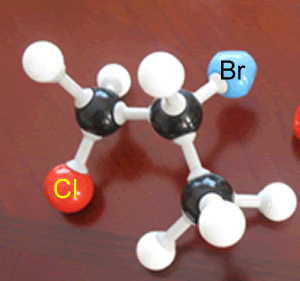
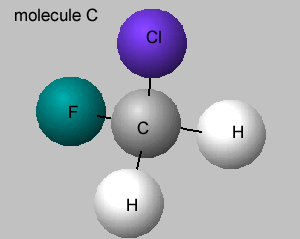
A student built the molecular model shown on the right of an organic molecule..
What is the name of this molecule?
How many optical isomers does it have?
Identify the chiral atoms.
Solution