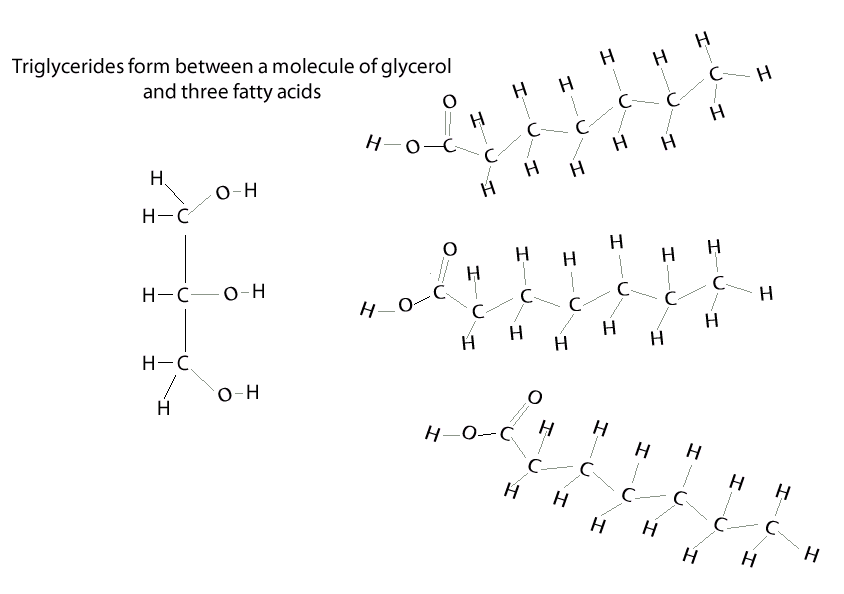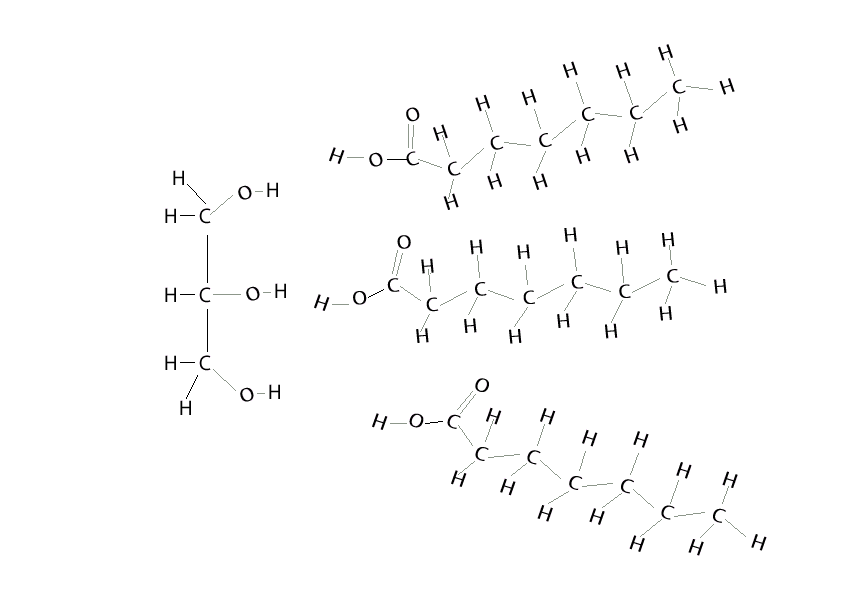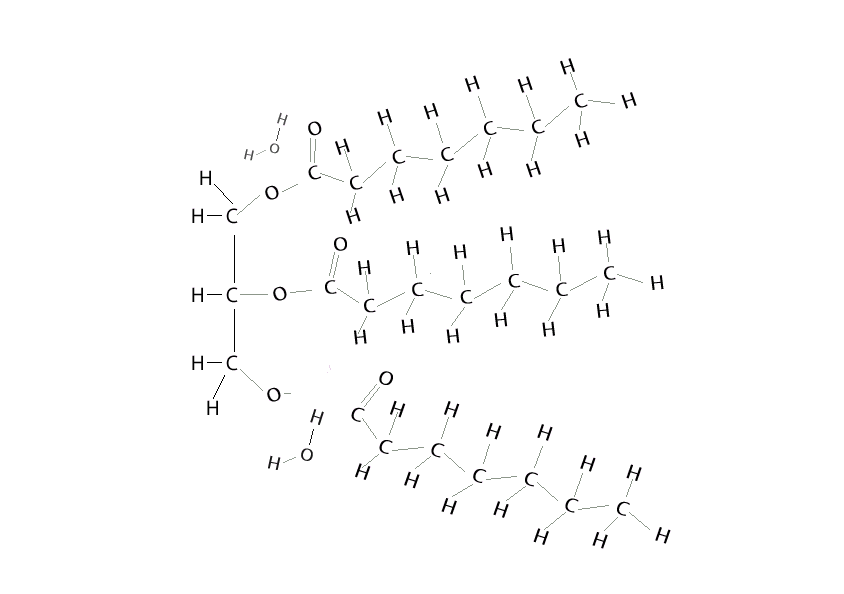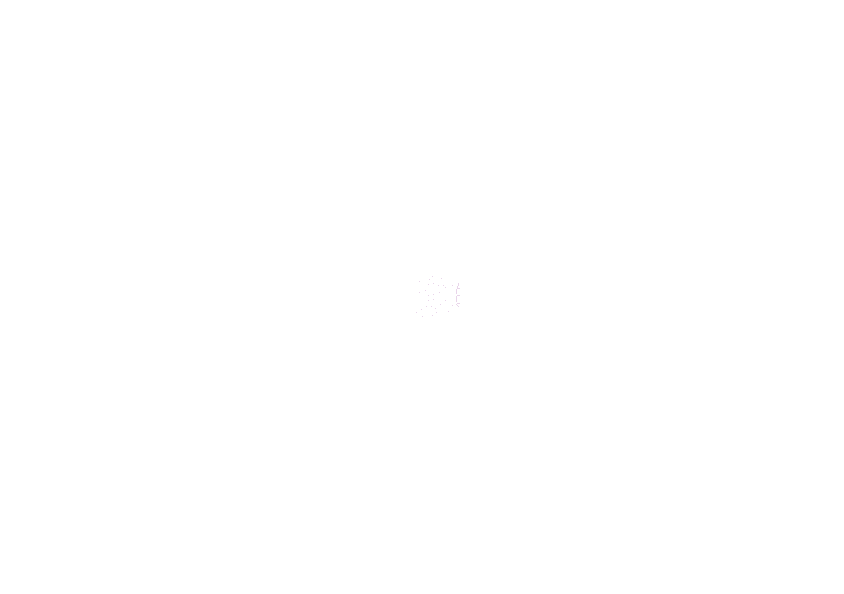Condensation reaction
Macromomolecules.
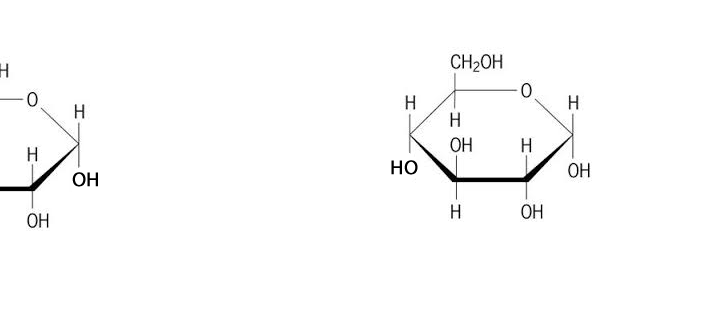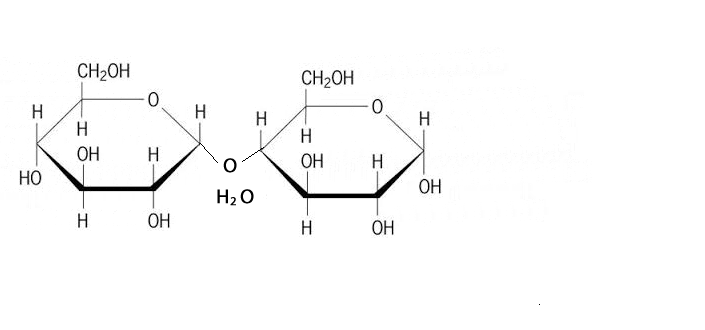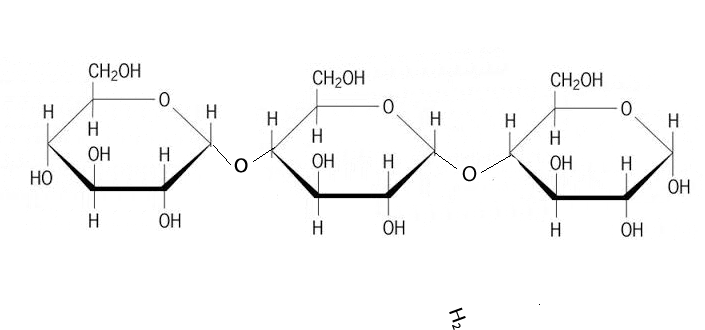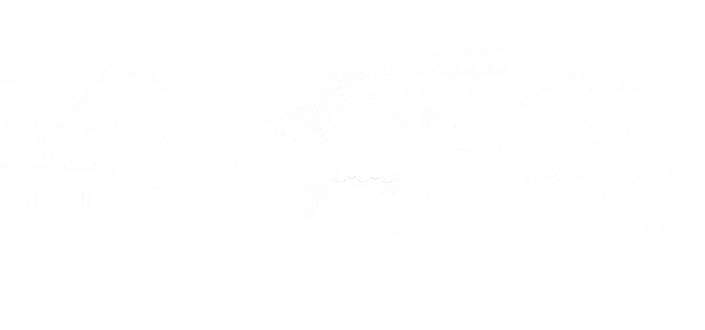
A condensation reaction is a chemical reaction where 2 molecules are joined together by a covalent bond to make a larger, more complex, molecule, with the loss of a small molecule. Water is often the small molecule that is given off at the creation of every covalent bond that links the two molecules together. Condensation reactions form all the biological macromolecules that we know, such as, proteins, fats, nucleic acid and carbohydrates.
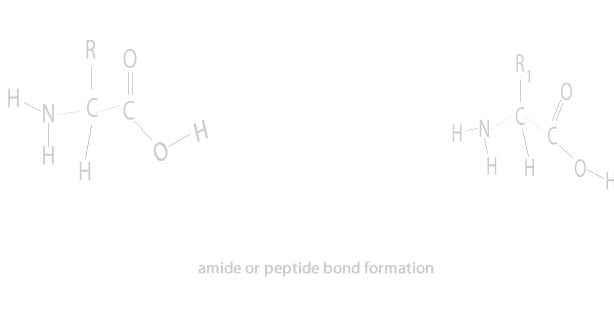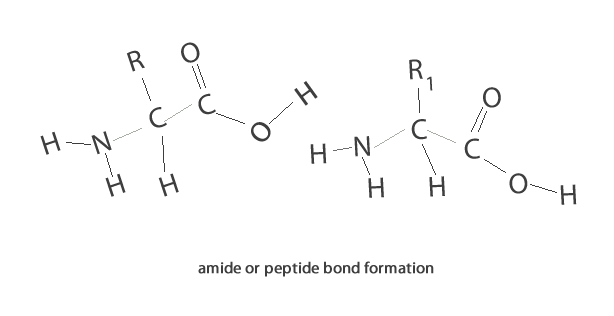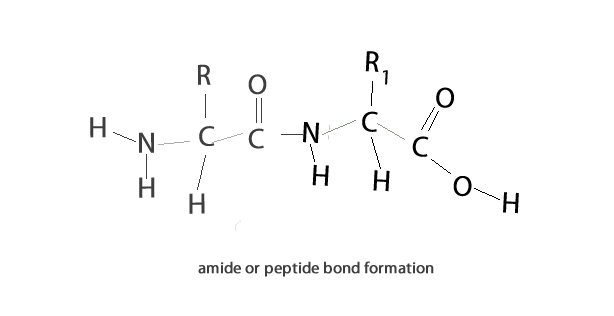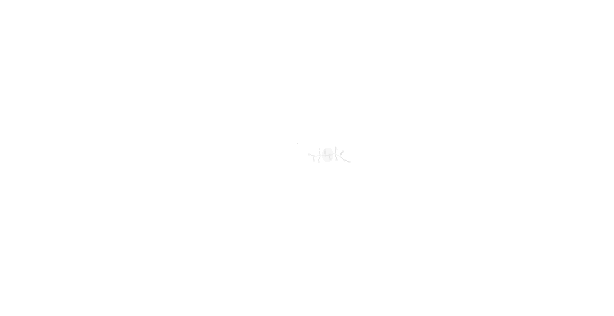
Below is an animation showing the formation of a triglyceride via a condensation reaction. Once again notice the expulsion of water at every link.