Each enzyme has a temperature range in which a maximum rate of reaction is achieved. known as the optimum temperature range for the enzyme.
Increased temperature initially increases the rate of the reaction (due to increased kinetic energy). However, enzymes:
- have an optimum temperature range in which they function effectively as catalysts;
- and an optimum pH.
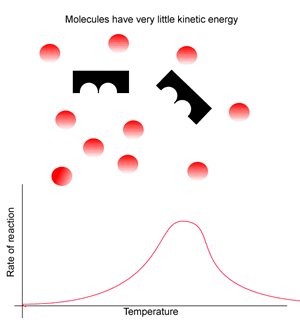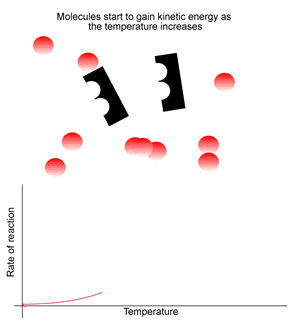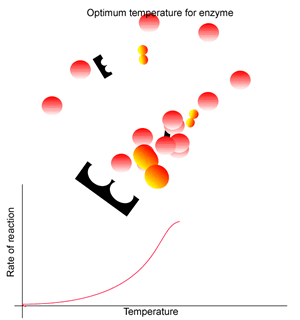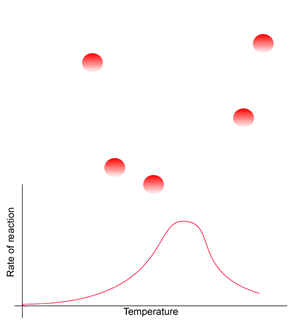
Consider the animation on the right. Generally, at low temperatures the rate of reaction is very low due to the lack of kinetic energy. In order for a reaction to proceed molecules must collide with enough force to cause chemical bonds to break.
At a certain temperature range the rate of reaction is optimal. Within this range substrate molecules have enough energy to move to and bind with the enzyme where they reaction is facilitated.
Beyond this optimal temperature range the reaction rate decreases as heat denatures the protein enzyme. Heat overcomes the many hydrogen bonds that contribute to the secondary and tertiary structures of the protein thus creating the unique shape of the protein.
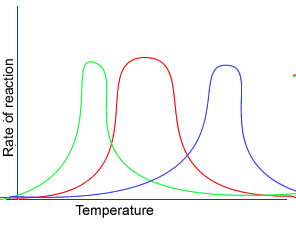
It is important to note that different enzymes may have different optimal temperature ranges. In the figure on the right the temperature optima of three different enzymes is shown.
The curve in green might represent an enzyme isolated from a shrimp that normally lives in the cold waters of Alaska. Thus its enzymes have evolved to work best at lower temperatures.
The curve in red might represents an enzyme isolated from a mammal.
Curve in blue might represent the temperature optimum of an enzyme present in a bacteria that normally lives in the hot springs of Yellowstone National Park. The enzymes from this bacteria would work best at temperatures that would normally denature enzymes isolated from mammals.
It is important to also note the shapes of the curves are also different.