Oxidation and reduction
Redox reactions
Sparklers are a fine example of oxidation/reduction (redox) reaction.

Click to see an activity on how to make sparklers
The sparklers above represent a quick rusting (oxidation) reaction between iron (Fe) and oxygen gas. It actually involves the transfer of electrons from the iron to the oxygen as shown in the animation on the right.
For all reactions involving electron transfer there is one reactant that takes the electrons called the oxidant and another that give them called the reductant.make sparklers.
Also note in the animaiton on the right that the number of electrons given equals the number of electrons taken.
Identify the oxidant in the animation on the right
Identify the reductant in the animation on the right
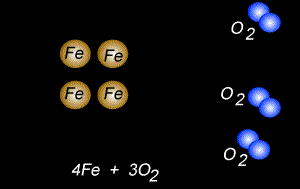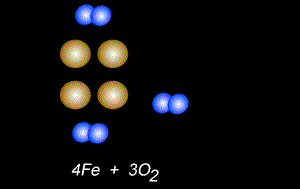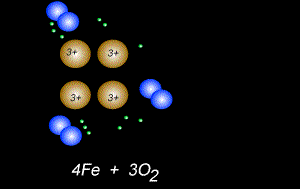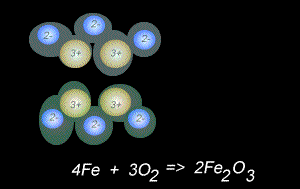

The fomation of rust is also a form of oxidation and takes place between oxygen gas and iron metal, according to the equation below.
4Fe(s) + 3O2(g) => 2Fe2O3(s)

When a compound such as iron oxide loses oxygen the process is known as reduction.
2Fe2O3(s)=> 4Fe(s) + 3O2(g)
Converting iron ore into iron, as shown on the right, is an example of a reduction reaction

.
Oxidation can also be described as the loss of electrons. While reduction is the process involving the gaining of electrons.
Although we have described oxidation and reduction separately they occur simultaneously. Reactions that involve oxidation and reduction are known as redox reactions. Redox reactions belong to a family of reactions that involve a transfer of electrons. Describing oxidation and reduction reactions as a gain and loss, respectively, of oxygen has limited use. A more precise definition is that oxidation is a reaction that produces electrons while reduction is a reaction that uses electrons. Redox reactions therefore occur as two half reactions, oxidation, electron producing reaction and reduction, electron using reaction.
The burning of magnesium ribbon is a reaction classified as a redox reaction. The oxidation and reduction reactions occur simultaneously. The overall reaction for the burning of magnesium is given below.
2Mg(s) + O2(g) => 2MgO(s)
But it can be separated into two half reactions the oxidation, where electrons are given, and the reduction, where electrons are used.
Mg(s) => Mg+2(s) + 2e-
O2(g) + 4e =>2O-2(s)
