Sparklers
Sparklers contain a mixture of metals which include aluminium, iron and magnesium. The mixture also contains oxidants. Oxidants are chemicals that produce oxygen when they are heated. The heat energy produced speeds up the oxygen molecules and causes them to collide quickly and violently with the metal atoms. The reaction now proceeds very quickly indeed.
Al = aluminium, Fe = Iron, Mg = Magnesium.
1) Write the two half equations for the formation of each oxide compound below.
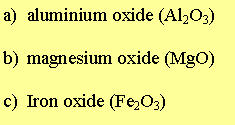
Activity
- Make your own sparklers.
