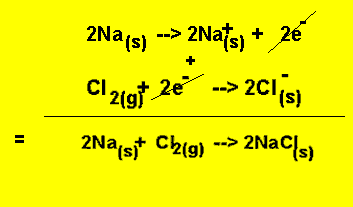Redox reactions
Half reactions
Take the reaction between sodium and chlorine gas.
2Na(s) + Cl2(g) = 2NaCl(s)
This redox reaction can be separated into two half equations as seen on the right.
The reaction below represents the oxidation reaction.
![]() (1)
(1)
The reaction below represents the reduction reaction.
![]() (2)
(2)
The electrons given in the oxidation reaction must equal the electrons used up in the reduction reaction. So just like simultaneous equations we find the lowest common multiple of the electrons. In this case the lowest common multiple is 2. We multiply equation (1) by 2 to get
![]() .
.
And now add the two equations to obtain the overall equation.