Electricity
production
from chemical reactions
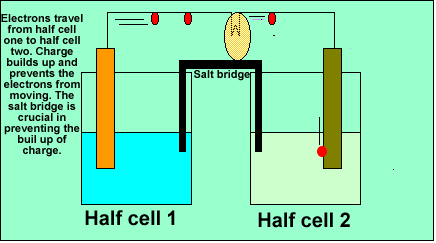
Batteries are made up of two separate compartments called half cells.
The electrons are forced from one compartment to another. The electrons
travel through the wire as they pass from one compartment to the other.
As electrons leave one compartment and go to the other, charge builds
up in each compartment and stops the electrons moving. A salt bridge
is used to minimise the build up of charge and keep the electrons flowing.
View the animation of the Elecrochemical cell .
After viewing
the animations answer the following questions.
1) What charge builds up in the half cell where the electrons go to?
2) What charge builds up in the half cell where the electrons come from?
3) What charged particles, from the salt bridge, flow into the half
cell where the electron go to?
4) What charged particles, from the salt bridge, flow into the half
cell where the electrons came from?
5) Knowing that similar charges repel each other while opposite charge
attract, suggest why the flow of electrons stops as the charge builds
up in each half cell.
*Electrons are negatively charged