The
modern day battery 
Modern day batteries are a sophisticated form of the old-fashioned galvanic cell. Instead of solution as an electrolyte the modern day batteries have a paste.
Two types of cells are in common use today, primary cells and secondary cells. Primary cells have a limited amount of chemical energy after it is exhausted the battery is discarded. Secondary cells on the other hand are rechargeable. An example of a secondary cell is the humble car battery. For a battery to be rechargeable the products from the reaction must stay on the electrodes. A car battery that has been shaken around has a limited life span because the products become dislodged from the electrodes and the reaction is unable to be reversed.
The lead acid accumulator
The car battery.
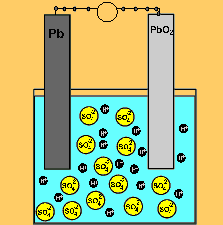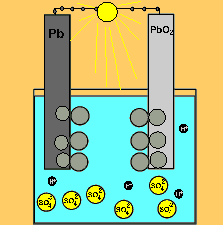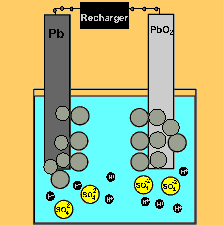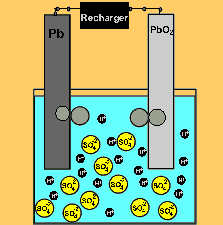
The supply of electrical energy from a recharger forces the reverse reaction to take place. Note how the products of discharging (lead sulfate) remain stuck to the electrodes. This enables the reverse reaction to take place. Note how the sulfuric acid is used up as the battery discharges. The pH will rise on discharging and decrease as the battery is recharged.