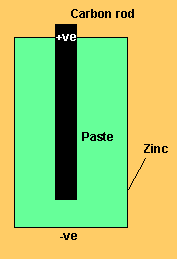
Solution
The reaction below represents the oxidation reaction which occurs at the anode or negative terminal
![]()
The reaction below represents the reduction reaction which occurs at the cathode or positive terminal
![]()
Adding the two reactions above will give the overall reaction occurring as the cell discharges.
![]()
Click to hide the solution