Hydrocarbons
The favourite child
of the Alkene family.
Ethene
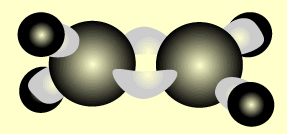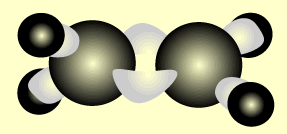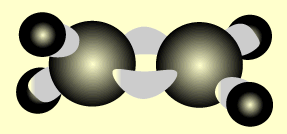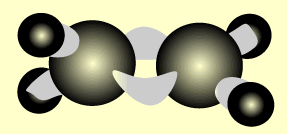
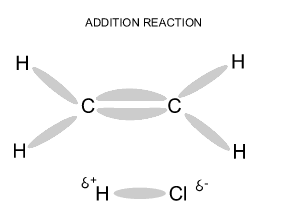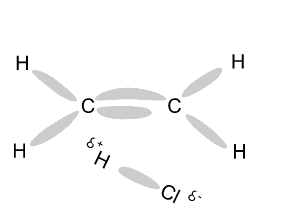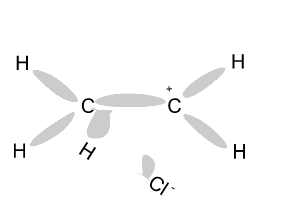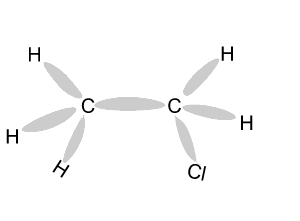
Name and write
the formula of the products
formed during an addition reaction between ethene and:
- H2
- Br2
- Cl2
Bromine (Br2) dissolves in water to form a brown coloured solution. Br2 solution is often used to test the presence of a unsaturated hydrocarbon.
The animation on the right shows a saturated hydrocarbon in test tube A and an unsaturated hydrocarbon in test tube B. The addition of Br2 solution reveals the presence of the unsaturated hydrocarbon by becoming clear. Br2 undergoes an addition reaction with the hydrocarbon. The Br2solution added to the unsaturated hydrocarbon will continue to turn clear until all the double bonds have reacted with the Br2.

The addition reaction of bromine with unsaturated hydrocarbons is extremely exothermic, as can be seen from the video on the right.
A C=C bond and a Br-Br bonds are broken while two C-Br bonds are formed.
What can you say about the energy in the bonds of the reactants compared to the energy in the bonds of the products?