Hydrocarbons
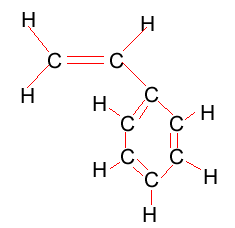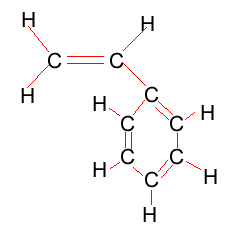
Ethene polymerisation
You can find this plastic in most kitchens as Cling Wrap.

The reaction to form polyethene is initiated by a free radical. A free radical contains an unpaired electron and is very reactive. This free radical attacks the double bond of ethene and bonds to the carbon atom of ethene. In the process it creates another free radical which attacks the double bond of a neighbouring ethene molecule. This process will become clear in the animation on the right.
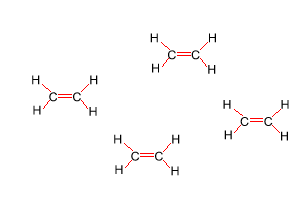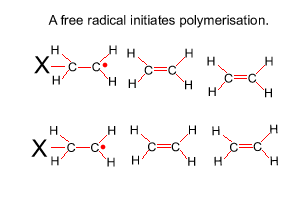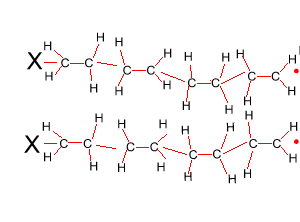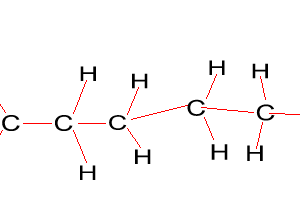
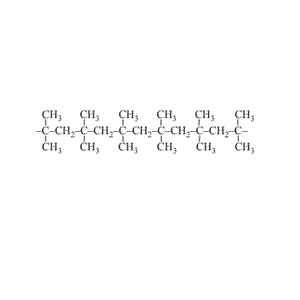
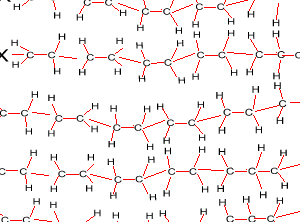
Look at the structure of polyethylene on the right. Describe the intermolecular and intramolecular bonding in polyethylene.
Explain why polyethylene exists as a solid while ethene is a gas at room temperature.
On the right is the structure of styrene. As you can see, styrene is ethene with a benzene ring replacing one of the hydrogen atoms. Notice how the double bonds on the benzene alternate position. Draw the repeating unit of polystyrene.
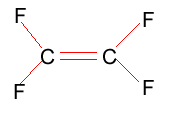
Tetrafluoroethylene is shown on the right. Polytetrafluoroethylene (PTFE) is best known as Teflon.
Teflon is best known for its uses in non-stick surfaces, such as frying pans.
Compare the intermolecular and intramolecular bonding in both polyethylene and PTFE. Suggest why PTFE can be used in high temperature situations, such as coating frying pans, where as polyethylene can not. Melting temperature of PTFE is 327oC, while that of polyethylene is between 110oC and 125oC.
Consider the addition polymerisation of but-2-ene, pictured on the right. Which is the semi-structural formula of the resulting polymer?
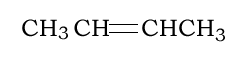
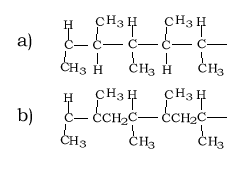
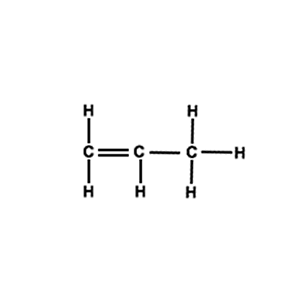
Draw the structural formula of a section of the polymer formed by the molecule on the right( propene).
Solution
Idnentify the monomer used to form the section of polymer shown on the right.
Solution