Reaction pathways of ethane and ethene are summarised in the diagram below. Click on the coloured arrows to see an animation of the reaction.
Red arrows represent addition reactions, blue arrows represent substitution reactions, the yellow arrow represents a condensation reaction and the green arrow is an oxidation reaction. Pink arrows indicate a redox reaction.
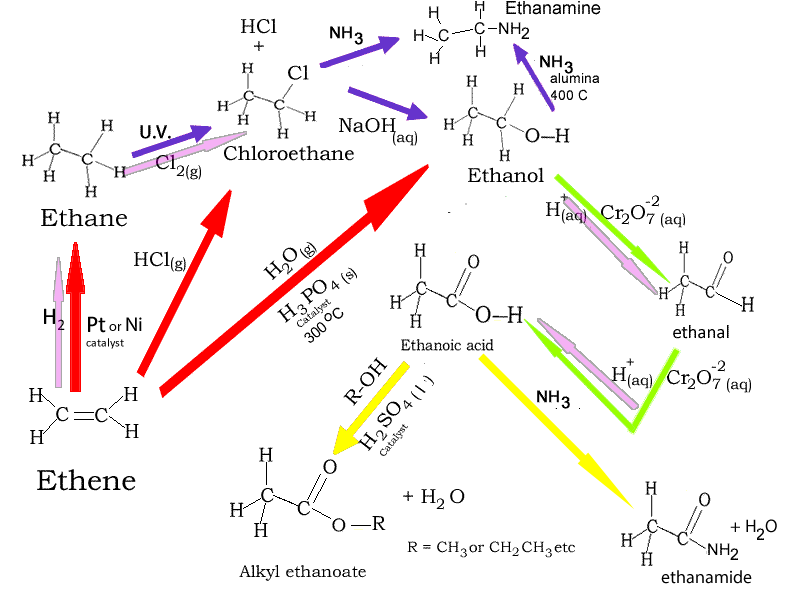
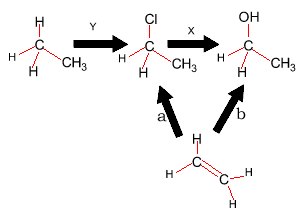
1) Consider the diagram on the right.
a) What substance is represented by "Y"?
b) What substance is represented by "X"?
c) What type
of reaction is "a"?
d) What else is needed for reaction "b" to proceed?
2) What is formed when a carboxylic acid and an alcohol react in the presence of sulfuric acid?
3) Sulfuric acid is needed when preparing esters from a carboxylic acid and an alcohol. The sulfuric acid acts
4) A polyester can be formed from the reaction between alcohol and carboxylic acids. What is necessary for the formation of a long ester polymer? Click to see the reaction
5) A compound with the following formula CH3CH2CH2OH is placed in an acidified solution containing Cr2O7-2 ions. What is the name of the product formed?
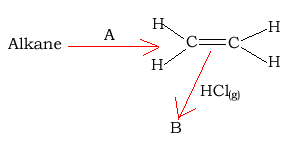
6) Consider the reaction pathways shown on the right.
a) Substance "A" is likely to be
b) Substance "B" is likely to be an
c) The name of substance "B" is
d) The reaction to form substance "B" is known as a
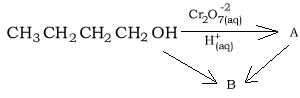
7) Consider the reaction pathways on the right.
a) Compound "A" is
b) Compound "B" is
c) Compound "C" is
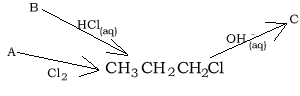
8) Consider the reaction pathways on the right.
a) The process labelled "A" is
b) Compound "B" is
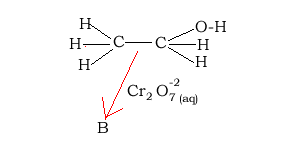
9) Consider the reaction pathway on the right.
The reaction that forms "B" is known as
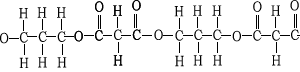
10) A section of a polyester
molecule is shown on the right. Which of the semi-structural fromulae,
below, represent the monomers?
a) HOCH2CH2CH2OH
b) COOHCH2COOH .
c) HOOCH2CH2COOH
d) OHCH2CH2OH
11) A monomer has the semistructural
formula HOCH2COOH. A polymer is formed from the reaction
of 1000 monomers.
a) What type of reaction does this represent?
b)The polymer is most likely to have a molecular mass close to