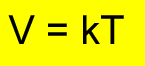Relationship between temperature and volume at constant pressure

To convert from Celsius to Kelvin we simply add 273 to the Celsius value.
Celsius + 273 = Kelvin
Click to try some exercises in converting Celsius to Kelvin.
In this expression K is a constant, V is volume in litres and T is temperature in degrees Kelvin.