Charle's Law exercises
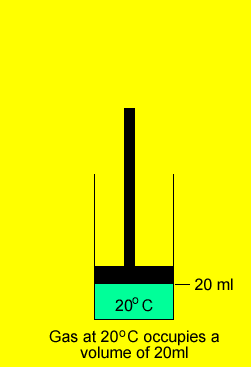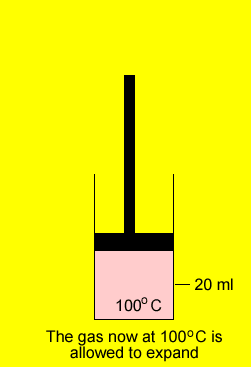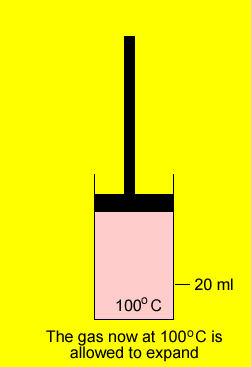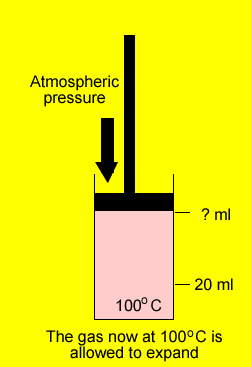
A gas was placed in a piston at 20oC and occupied a volume of 20ml. The gas was then heated to 100oC and allowed to expand under constant pressure. What is the final volume of the gas.
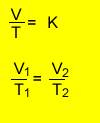
V1 = 20ml = 0.02L
T 1 = (20 + 273) =293oK
V2 = ?
T 2 = (100 + 273) =373oK
=>0.02/293 = V2/373
=>(0.02/293) X 373 = V2
=>25.5ml =0.0255L= V2
1) A sample
of air at 40oC is placed in a piston, similar to the one shown
above, and occupies a volume of 10ml. The sample was heated to 240oC
and the piston allowed to expand under atmospheric pressure. What was
the final volume of the gas inside the piston?
