Some chemicals are acids and they react with the water to make it acidic. The pH is the way in which we measure the acidity of the water. The pH scale goes from 1 to 14. A pH of 1 is very acidic and a pH of 14 means that not much acid is present at all. Some times we use chemicals called indicators that change colour at different pH levels. Universal indicator is one such chemical. Below are the colour ranges for the pH levels.

There is another group of chemicals called bases. These react with acids to neutralize them. At pH levels above 7 there is more base in the water than acid. At pH of 7 there is equal base and acid in the water and the water is said to be NEUTRAL.
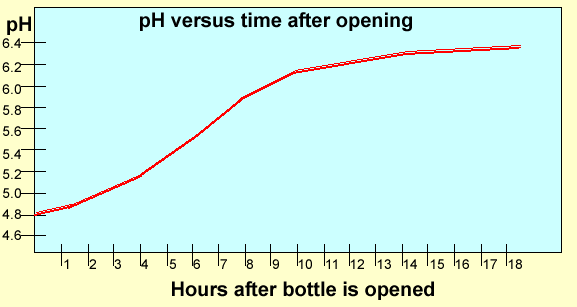
Carbonated
drinks have carbon dioxide gas dissolved in them. This gas makes the
water very acidic. As the drink is left open carbon dioxide escapes and
the drink becomes less acidic over time. Below is a graph of the pH of
a particular brand of Soda Water at 25C.

Explain.