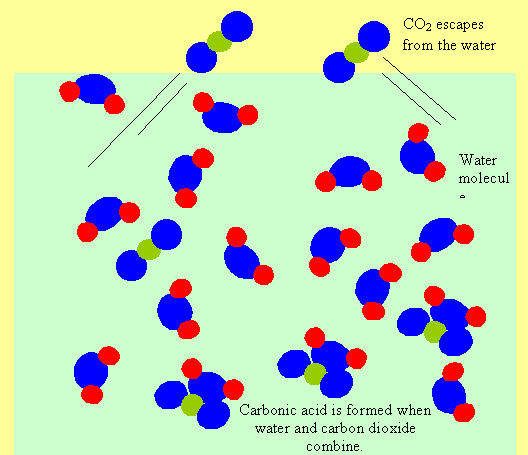
When carbon dioxide gas is dissolved in water it forms an acid called carbonic acid. This makes the water acidic. When a bottle of carbonated drink is opened the carbon dioxide gas escapes with a fizz. As the gas escapes, the water gets less and less acidic. We can measure how long the bottle was open for, by measuring the acidity of the liquid.

Chemicals called indicators are used to measure how acidic a liquid is. The indicator changes colour depending on the level of acidity. Above are 5 samples of Soda Water. Sample "A" is a fresh sample straight from the bottle. Sample "B" was left out for 30 minutes, while "C" was left for 1 hour, "D" was left out for 4 hours and "E" was left out for 12 hours.
As the liquid is left standing the carbon dioxide escapes. Design an
experiment to see if carbon dioxide escapes faster from warm drinks
than cold drinks. Plot your results on a graph.
* Hint use Universal Indicator and draw a graph of time versus pH.
Why does a warm bottle fizz loudly when it is opened than a cold one?