
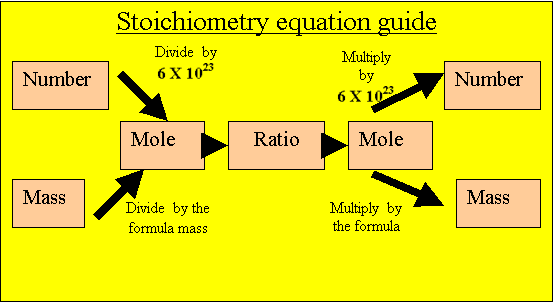
Convert the mass of oxygen gas into moles of oxygen gas.
32 grams/ 32 atomic mass units.
= 1 mole of oxygen molecules
ONE mole of oxygen molecules are present to react.
Caclulate the mole of aluminium oxide that will be produced
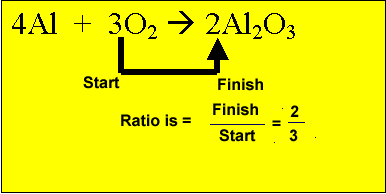
from the oxygen present. Multiply the mole of oxygen by the ratio.
2/3 X 1 = 2/3 mole of aluminium oxide.
Calculate the mass of aluminium oxide
Multiply the moles of aluminium oxide by its formula mass.
2/3 X 102 = 67 grams.
|
Limiting and excess reactants
|
|
When we combine two or more reactants there is usually too much of one and not enough of the other. Obviously the reaction will stop when one reactant is completely used up. Three scenarios exist a reactant can be in: excess, where there is obviously too much of the reactant present and not all will be used up; limiting, where the reactant will be used up completely before the other reactant is used up; The right ratio (proportion) with all other reactants and all will be used up in the reaction. So the amount of product formed depends on the limiting reactant. When the limiting reactant runs out the reaction will stop. It is similar to a recipe where the cooking stops when an essential ingredient runs out. So how do we deal with excess and limiting reagents?. We can best explain this with an example. Aluminium reacts with oxygen according to the reaction below. 4Al + 3O2 => 2Al2O3
We now use the oxygen to work out the mass of aluminium oxide produced.. |
|
Double Click anywhere in the green region of the document to see the working out. Click on the squares inside the diagram to reveal more information. Double click to remove the information. |