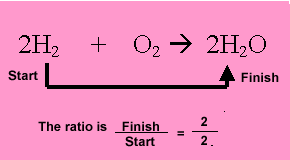
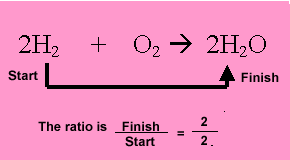
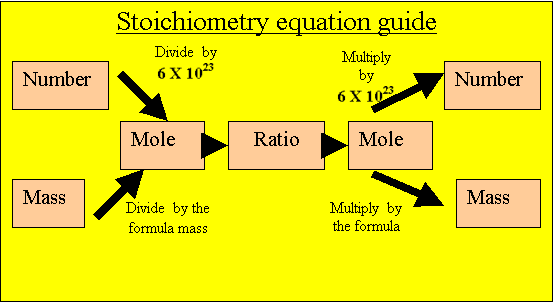
Convert the mass of hydrogen gas into moles of hydrogen gas.
8 grams/ 2 atomic mass units.
= 4mole of hydrogen molecules
4 mole of hydrogen molecules are present to react.
Calculate the mole of water that will be produced from the
hydrogen present. Multiply the mole of hydrogen by the ratio.
2/2X 4= 4mole of water.
Calculate the mass of water
Multiply the moles of water by its formula mass.
4 X 18 = 72 grams.
|
Limiting and excess reactants exercise
|
|
Hydrogen reacts with oxygen to form water according to the equation below. 2H2 + O2 => 2H2O
We now use the hydrogen to work out the mass of water produced. |
|
Double Click anywhere in the green region of the document to see the working out. Click on the squares inside the diagram to reveal more information. Double click to remove the information. |