Solubility curves- separation of mixtures
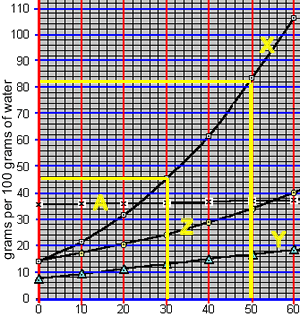
An 82 gram sample of compound "X" was accidentally mixed with 4 grams of compound "Z". Both solutes move freely in solution. As the temperature is reduced one of these compounds ("X") will precipitate out as shown below.
The mixture was then dissolved in 100 grams of water and heated to 50 oC until all the solute was dissolved. According to the solubility curve on the right this represents a saturated solution of compound "Z" and an unsaturated solution of compound "Z".
What mass of compound "Z" must be added to the 100 grams of water to create a saturated solution of compound "Z" at 50 oC?
The solution was then cooled to 30 oC to precipitate element "X" only. 36 grams of compound "X" was precipitated.
Why does sugar crystallise out of honey when it is cooled?
1) A mixture consisting of 40 grams of compound "X" and 40 grams of compound "Z" was given over to Stephen. Which of the following processes would yield a pure sample of compound "Y"?