The solubility of gases decreases as the temperature of the water increases as shown by the solubility curves on the right.
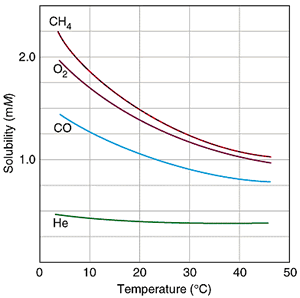
Increasing the temperature causes the kinetic energy of the molecule to increase. The higher kinetic energy causes the molecules to move about faster breaking intermolecular bonds and escaping from solution.
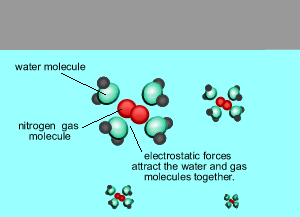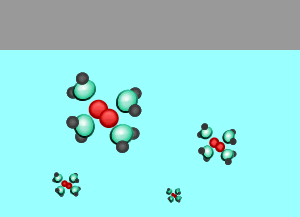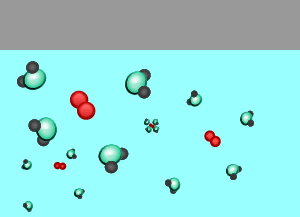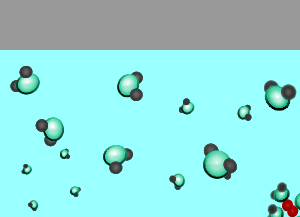
As the temperature rises the amount of gas that escapes the solution increases.
1) Which one of the following options best explains why a warm can of soft drink has more fizz when opened than a cold can.
2) What will happen to the air at the surface of the liquid inside a can of carbonated soft drink as it is heated?
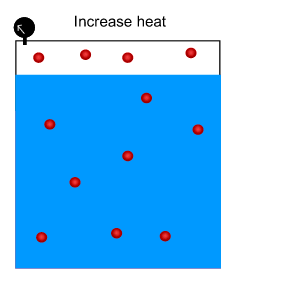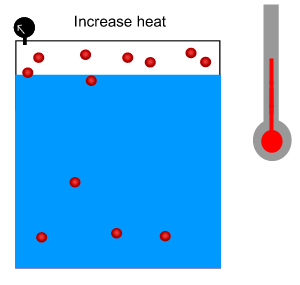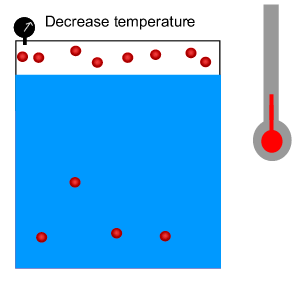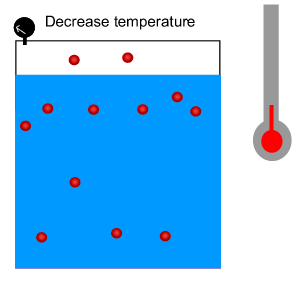
3) The Green House Effect is due to a build up of carbon dioxide in the atmosphere. Oceans dissolve a great deal of carbon dioxide. Scientists fear that carbon dioxde will be pumped out of the oceans and back into the atmosphere as the Earth warms up. Why?