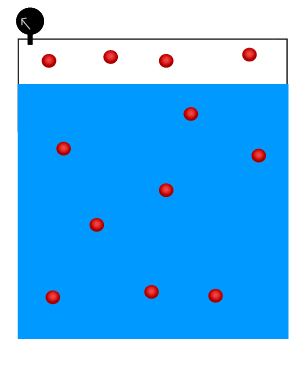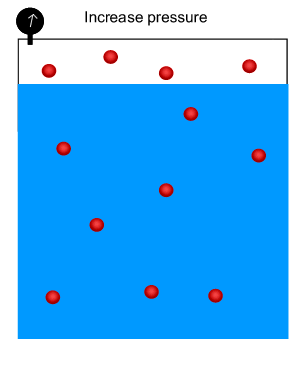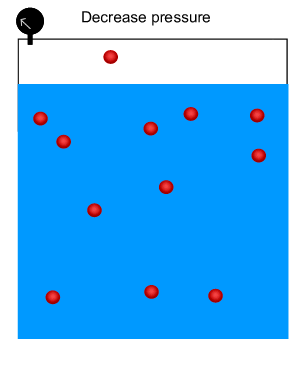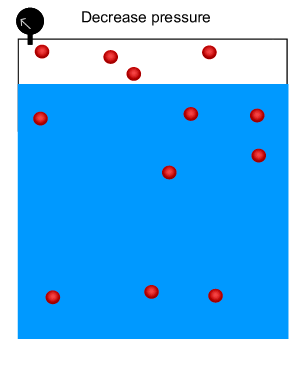
Solubility of a gas increases as the pressure of that gas above the surface of the liquid increases. In other words, at high pressure the gas is forced into the liquid and the can therefore hold more gas than at a lower pressure.
As shown in the animation on the right, the number of gas molecules above the surface of the liquid decreases at high pressure while the number of gas molecules in the liquid increases.
Carbonated drinks are bottled under high pressure to increase the amount of carbon dioxide dissolved in the drink. When the bottle is opened, the pressure above the solution decreases. Click to see this phenomenon. When the bottle is opened, the solution effervesces and some of the carbon dioxide bubbles out.


1) Deep sea divers breath a mixture of helium and oxygen. Helium is only one fifth as soluble in blood as nitrogen. How does this mixture assist in preventing the bends?
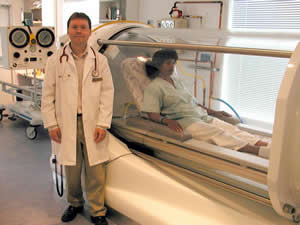
2) Divers suffering from the bends are placed in a high pressure vessel. How does this help?
3) Sports injuries often lead to hypoxia (low oxygen concentration) of injured tissue. This is overcome by a hyperbaric chamber where the patient breathes oxygen at high pressure. Explain how this works.