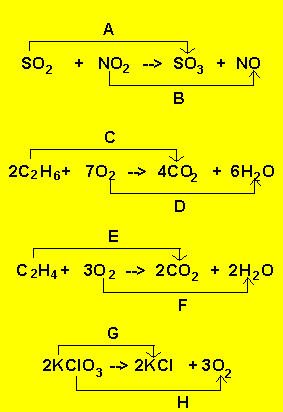
|
"A" is an oxidation
process because
a) Oxygen is added
b) Sulfur goes from an oxidation state of 4+ to 6+
"B" is a reduction
reaction because
a) Oxygen is lost
b) Nitrogen goes from an oxidation state of 4+ to 2+
"C" is an oxidation
reaction because
a) Oxygen is gained and hydrogen lost
b) Carbon goes from an oxidation state of -3 to 4+
"D" is a reduction
reaction because
a) Oxygen is lost and hydrogen gained
b) Oxygen goes from an oxidation state of 0 to -2
"E" is an oxidation
reaction because
a) Oxygen gained and hydrogen lost
b) Carbon goes from an oxidation state of -2 to +4
"F" is a reduction
reaction because
a) Oxygen is lost and hydrogen gained
b) Oxygen goes from an oxidation state of 0 to -2
"G" is a reduction
reaction because
a) Oxygen is lost
b) Chlorine goes from an oxidation state of +5 to -1
"H" is an oxidation
reaction because
a) Oxygen goes from an oxidation state of -2 to 0
|