Identifying redox reactions
All redox reactions are made
up of an oxidant and a reductant.
Oxidants cause oxidation of other species and in the process are themselves
reduced. Oxidants are repsonsible for taking electrons from other species
anf therefore oxidising them, but when they take the electrons they
are reduced. Reductants work in exactly the same way but in reverse.
They cause other species to reduce and in the process are themselves
oxidised.
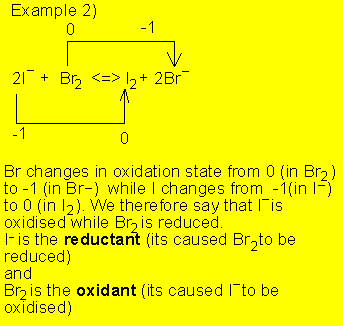
Let's see some examples
![]()
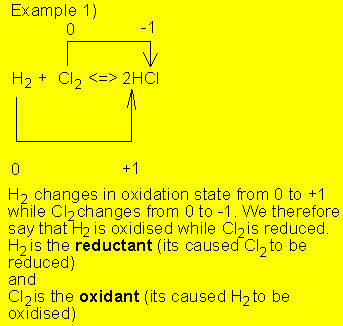
![]()