Metal Y is less reactive than metal Z. While both X and Y are more reactive than Z
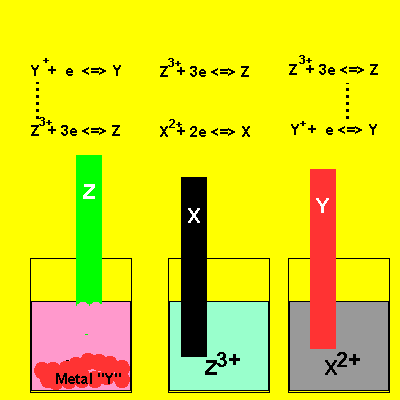
The metals appear in the following order in the reactivity series.
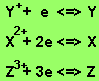
The order of increasing reactivity
is Y, X, Z
Hide solution
Metal Y is less reactive than metal Z. While both X and Y are more reactive than Z
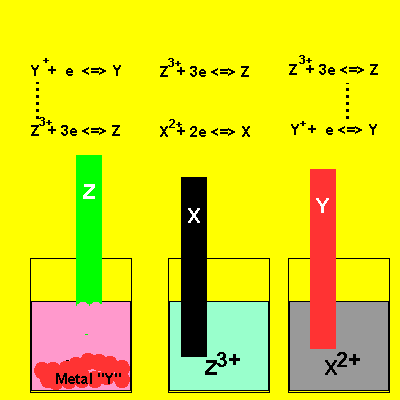
The metals appear in the following order in the reactivity series.
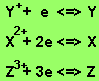
The order of increasing reactivity
is Y, X, Z
Hide solution
|
Advanced exercises part 2 |
|
|
A chemist working for RAZMAN'S Mining Co. conducts the experiment depicted in the animation on the right. Irene places metals "Z", "Y" and "Z" in solutions of their nitrate salts. The results are shown . Metal "Z" dissolves when placed in a nitrate salt of metal "Y" and solid "Y" appears at the bottom of the beaker. Nothing happens when "X" is placed in a nitrate solution of metal "Z". No reaction is observed when "Y" is placed in a nitrate solution of meal "X" . State the metals in order of increasing reactivity. ie in order from lowest to highest strength reductant. Solution |
|