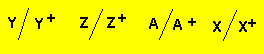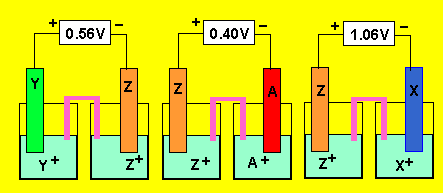
a) In the cell constructed
by the two half cells![]() ,
what happens to the mass of each electrode?
,
what happens to the mass of each electrode?
Electrode "Z" decreases in mass while electrode "Y" increases.
b) From the information given, what order do the half cells appear in the electrochemical series?
Since electrode
"Z" forms the anode in the first cell it is being oxidised by "Y".
"Y" is therefore higher on the electrochemical series than "Z".
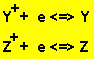
Now "Z" ions oxidize metal "A" and metal "X".
So both metal "A" and "X" are below Z on the electrochemical
series. Looking at the EMFs of the cells "X" is lower than "A".
So the series looks like this
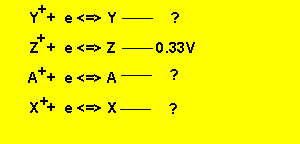
c)
If the half cell potential of ![]() is 0.33V what is the cell EMF of a cell constructed from the two half cells
below?
is 0.33V what is the cell EMF of a cell constructed from the two half cells
below?
![]()
First we must
work out the half cell potentials using the given cell EMFs and the half cell
potential of ![]() .
Follow the animation below.
.
Follow the animation below.
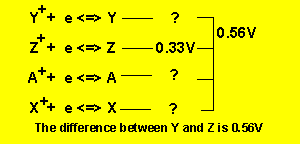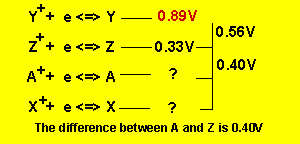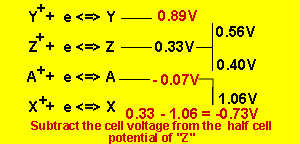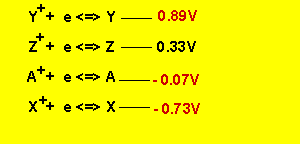
We arrive at the series table
listed below.
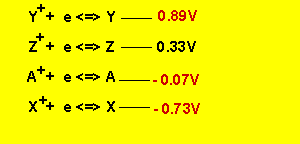
The cell EMF of ![]() is
0.33 - - 0.07 = 0.4V
is
0.33 - - 0.07 = 0.4V
Hide solution