Rates of reaction
Catalysts
Catalysts act by allowing the reactant particles an alternative route with a lower activation energy. Activation energy is the energy required to break the chemical bonds of the reactants. Catalysts can significantly increase the rate of reaction without an increase in temperature or concentration of reactants. During chemical reactions the catalyst is not used up.
Two types of catalysts exist organic and inorganic. Organic catalysts are better known as enzymes and their role is to catalyse and regulate specific reactions in living organisms. Inorganic catalysts are often metallic compounds with a high surface energy. This high surface energy assists in the breaking of chemical bonds present in the reactant molecules. Click to see a simple animation
An organic catalyst has a small portion of its surface with a special shape. This part of the surface is known as the active site. The active site holds the reactant molecules in a certain orientation that makes them vulnerable to bond breaking during mild collisions. This decreases the amount of energy (temperature) required to break the chemical bonds.
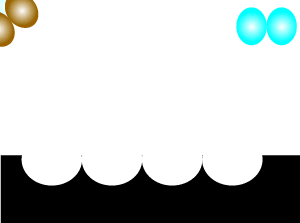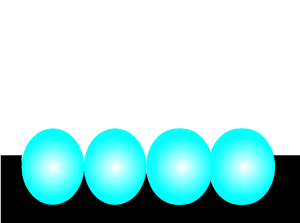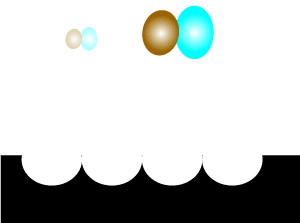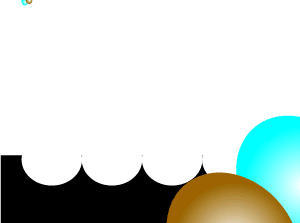
Consider the animation on the left. A catalyst, shown in black, is present. Most of the reactant particles do not have enough energy, known as activation energy, to collide with such force as to break bonds and inititate a reaction. However the catalyst offers an alternative pathway that enables more reactant particles, that do not have the necessary kinetic energy, to react.
It is important to note that a few reactant particles do have enough energy to collide and initiate a reaction without the use of a catalyst. However their numbers are so low that a reaction can not be sustained.
In the absence of a catalyst the reaction is too slow. Click to see the uncatalysed reaction.