Fractional
distillation
The fractionating tower |
 |
Lets see how
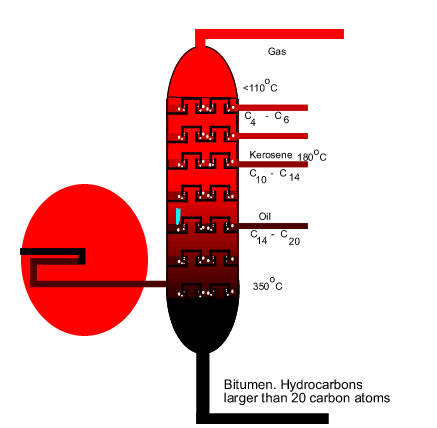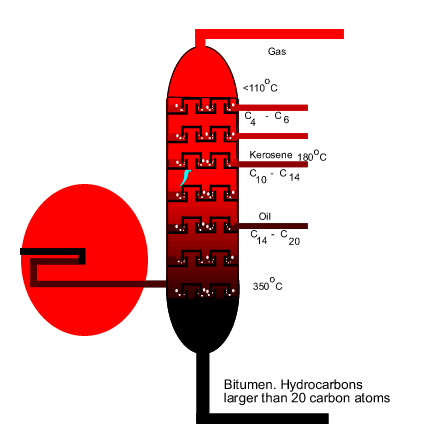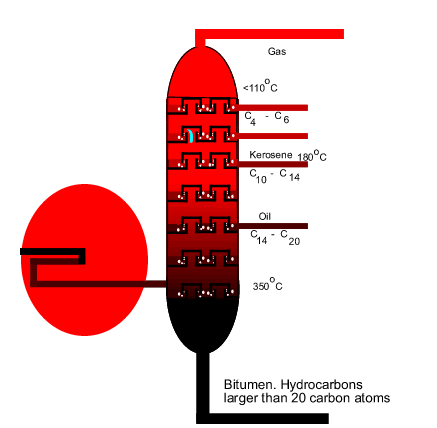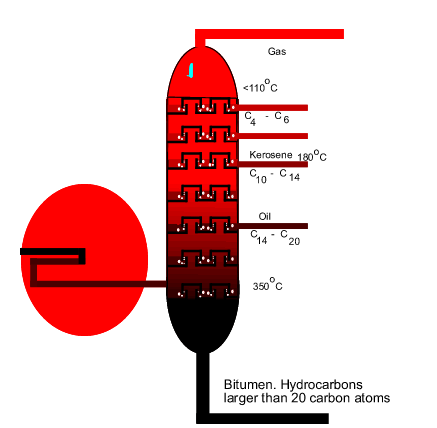
the process of fractional distillation works. The diagram on the right shows
an internal view of a fractionating tower. The blue arrow reveals the
path that gaseous compounds are forced to take as they move upwards.
|
| Crude oil enters
the tower at approximately 375oC. At this temperature the oil
becomes a hot mixture of liquid and gas. As this hot mixture enters the
tower the liquid and gaseous compounds quickly separate. Liquids sink to
the bottom of the tower, where they are collected, while the gaseous compounds
rise upwards. As seen in the diagram above, the temperature in the tower
gradually decreases as the gases rise. Different gas compounds condense,
at different temperatures, as they move up the tower and are collected. |
|
|
|
The tower contains
many horizontal trays with hundreds of bubble caps. These
bubble caps prevent the easy rise of gases through the column and force
the rising gas mixture to bubble through condensed liquid in the tray.
The bubble caps move up and down as gas pressure increases.
The animation on the left shows
how the bubble caps work, while the arrow reveals one of the paths that
the hot gas mixture is forced to take as it rises.
|
| Compounds, in the
rising gas mixture, that have the same boiling point as the condensed liquid
in the tray will condense and be collected. As the gas mixture moves up
the tower compounds with lower boiling temperatures will be collected. |
| Not only is the
hot gas mixture separated by boiling temperature but also separated by molecular
size. As we discovered earlier, alkanes are symmetrical molecules that are
held together by weak intermolecular forces called dispersion
forces. The strength of these relatively weak forces depends
on the size of the molecule. Since the boiling temperature depends on the
strength of the intermolecular forces, separating the molecules in order
of boiling point will also separate them in order of size as well. As seen
in the diagram above, compounds that condense further up the tower have
relatively lower molecular mass than those that condense lower down the
tower. In fact the hydrocarbons that are found at the bottom of the tower
are so large that they are often used as bitumen for lining road surfaces. |
| |
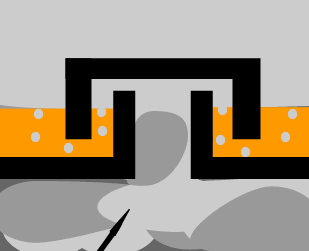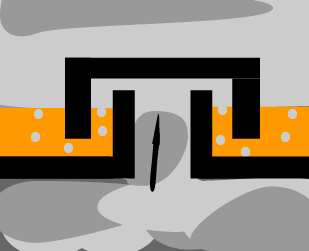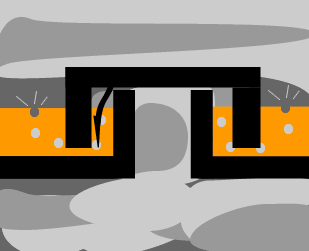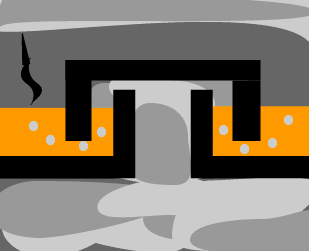
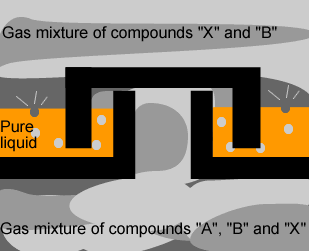
The diagram on the
left shows the hot mixture of gas rising through the trays in the tower.
Identify the pure liquid in the tray and give reasons why you selected this
compound. |
|
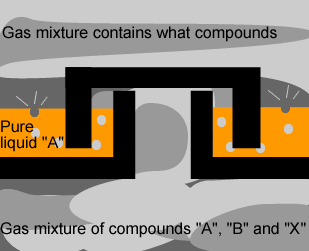
The diagram on the
left shows the hot mixture of gas rising through the trays in the tower.
What is the composition of the gas mixture above the tray? Explain. |
|
The fuel tanker
contains octane, which is a major component of petrol and rolls along the
road lined with bitumen. Describe where, on the fractionating tower would
we expect to find octane and bitumen. Give reasons. |
|
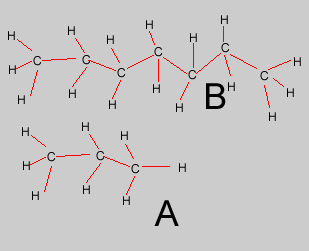
Compound "A"
is expected to condense higher up the fractionating tower than compound
"B". Why? |
|
|