Two types of cracking processes are used in industry.
The first is called thermal
cracking. In the absence of air, steam and raw material
are mixed and heated to temperatures around 800oC inside a
furnace. During this process, lighter unsaturated hydrocarbons are produced
from the thermal decomposition of heavier saturated hydrocarbons. Two
examples are given below. The gases are in the furnace for less than one
second in order to prevent continued decomposition of ethene into carbon
(coke). As the products leave the furnace they are quickly liquefied at
temperatures around -100oC and separated by fractional distillation.
Carbon dioxide and hydrogen sulfide are unwanted byproducts of thermal
cracking. These gases must be removed before they escape into the atmosphere
by reaction with sodium hydroxide solution.
2NaOH(aq) + H2S(g) => Na2S(aq) + 2H2O(l)
2NaOH(aq) + CO2(g) => Na2CO3(aq) + H2O(l)

C2H6(g) => C2H4(g) + H2(g)

C3H8(g) => C2H4(g) + CH4(g)
The second method
is known as catalytic cracking. In this process
a catalyst, zeolite, is used to crack heavy
fractions at temperatures around 500oC. The products are a
mixture of saturated and unsaturated hydrocarbons. Octane is a valuable
product and is used as a major component of petrol. The other products
are used as feed stock for thermal cracking to yield more ethene.
The benefits of catalytic cracking as opposed to thermal cracking include:
- greater control of the range
of end product;
- lower temperatures are used (450oC)
Below is a diagram for the overall cracking process.
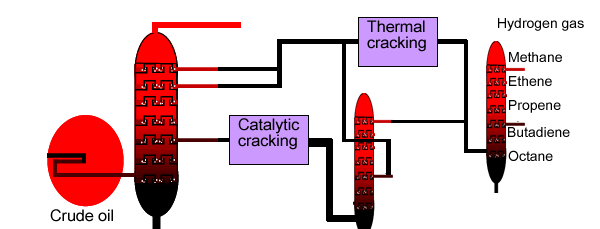
Fractional distillation is used to separate the products of thermal and catalytic cracking.
You can crack hydrocarbons at home using a candle. Click to see a 120 kb video. The paraffin wax molecules are too large to vaporise easily at the temperature of the flame. Something else must have been present in the smoke that was more flammable than paraffin wax vapour. Cracking occurs to produce smaller hydrocarbons that ignite in the flame of the candle.

What is the purpose of the flame?