
Vegetable oils are sometimes used to provide the fatty acids for biodiesel formation. A number of these oils are listed in the table on the right.
1) Why, in the harsh European winter, is a low melting temperature ideal?
The fuel must by in liquid form to flow through the system and into the ignition chamber. A low melting temperature ensures the fuel remains in liquid form and therefore able to be pumped through the fuel system, even in very low temperatures.
| Name of oil | Number of carbons | Number of double bonds | Melting temperature |
| Caprylic | 8 | 0 | 16.5 |
|---|---|---|---|
| Lauric | 12 | 0 | 44 |
| Palmitic | 16 | 0 | 63 |
| Oleic | 18 | 1 | 16 |
| Linoleic | 18 | 2 | -5 |
| Linolenic | 18 | 3 | -11 |
2) What is the trend between melting temperature and number of double bonds? Explain
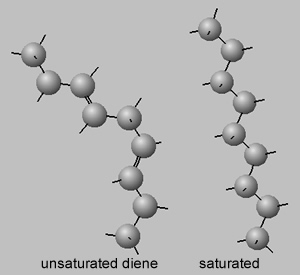
Melting point are linked to the the strength of the intermolecular bonds that exist between molecules. These intermolecular bonds are electrostatic in nature and the charges need to be in close proximity to generate a reasonable attraction. Double bonds tend to kink the molecule, as shown on the right. Molecules which are bent cannot pack close to each other along the length of the molecule and hence charges on the surface of the molecule are unable to get close to each other to generate a reasonable force of attraction. Saturated molecules, however, are straight and pack in very close to each other.
3) Why does the melting temperature increase as the number of carbon atoms in the molecule increases?
Melting temperature is directly related to the strength of the intermolecular forces between molecules. The bigger the molecule the greater the intermolecular forces generated by dispersion forces hence the greater the melting temperature. Since hydrocarbons have dispersion forces only acting between molecules the bigger the molecule the greater the strength of the intermolecular bonds and hence the greater the melting temperature.
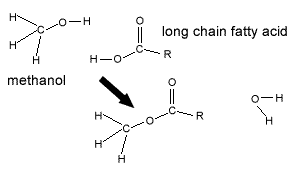
A process known as transesterification is commonly used to produce biodiesel. Chemically, transesterification is the process of exchanging the alkyl group of an ester with another alkyl group, from a different alcohol.
Vegetable oil contains fatty acids bonded to glycerol to form triglycerides.
In the case of biodiesel, a vegetable oil ester is combined with a simple alcohol, methanol, and a catalyst (NaOH), resulting in the breakup of the triglyceride ester to form glycerol and three methyl esters (biodiesel) as shown on the right.
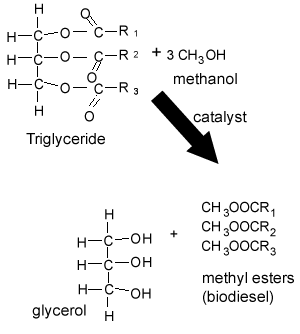
4) Rudolf Diesel invented the diesel engine which was initially designed to run on vegetable oil. Vegetable oil is composed of triglycerides, as shown on the right. In colder climates vegetable oil is not a viable fuel as it becomes very viscous and even solidifies in the fuel line. Some vehicles have heating coils that preheat the vegetable oil before it enters the fuel lines. Why is it necessary to convert the vegetable oil into biodiesel (methyl esters)?
Triglycerides are big molecules made up of three fatty acids attached to a glycerol molecule. Since they are big molecules they have high melting points and tend to solidify at low temperatures. Biodiesel, however, is made of smaller molecules known as fatty acid methyl esters, as shown on the right. Biodiesel has a low melting point and is not as viscous as the triglycerides. This why biodiesel, instead of vegetable oil, is used in cars.
.