Hydrocarbons
The family of alkanes
The compounds in this homologous series are characterised by single covalent bonds between hydrogen and carbon. Atoms can rotate about a single covalent bond. Click to see an animation.
Alkanes are insoluble in water and react only under the right conditions. We are all familiar with petrol and its explosive properties in the presence of oxygen and a flame. Click to find out more about some of the reactions of alkanes.
Name |
Molecular
formula |
Structural
formula |
Diagram |
Methane |
CH4
| CH4 Click to see the symmetry of methane | 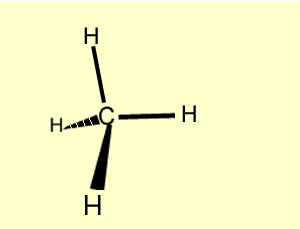 |
Ethane |
C2 H6
| CH3CH3
| 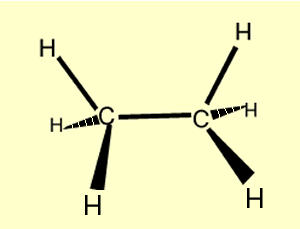 |
Propane |
C3H8
| CH3CH2CH3 | |
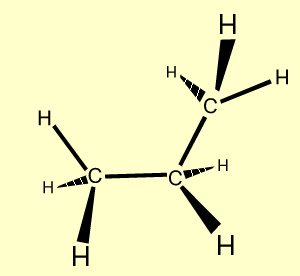
n-butane |
C4H10
| CH3CH2CH2CH3
|
Click to see a model of butane |
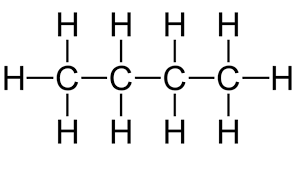
n-pentane |
C5H12
| CH3CH2CH2CH2CH3
| |
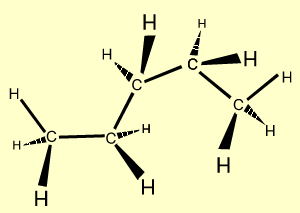
n-hexane |
C6H14
| CH3CH2CH2CH2CH2CH3
| 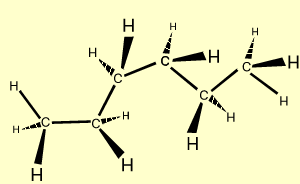 |
Why do the molecules,
represented diagrammatically, appear to be twisted?
What is the formula of n-heptane?
Look at n-butane. Are there any other ways of drawing this molecule?
(look at isomers)
Below is a table of the properties of some of the alkanes. Explain any
noticeable trends.
Name |
Boiling
point oC |
Physical
state at room temperature |
Methane |
-162 |
Gas |
Ethane |
-89 |
Gas |
Propane |
-43 |
Gas |
Hexane |
69 |
Liquid |
Heptane |
98 |
Liquid |