Acid and base
pH
pH is a scale that ranges from 1 to 14. Midway through this scale is the number 7 and a pH of 7 indicates a neutral solution. Neutral solutions have either no base or acid present or equal amounts of acid and base. A pH less than 7 indicates an acidic solution while a pH of more than 7 indicates a basic solution.
A pH close to 1 indicates a very strong acid while a pH just below 7 indicates the presence of a weak acid. Strong bases on the other hand have a pH close to 14 while weak bases have a pH just above 7. See the chart below. Scan the image for more detail.
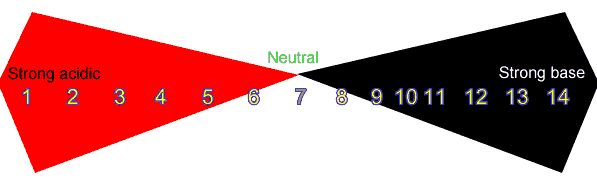

According to the chart the solution on the right has a pH of 7.5. This solution must be
The solution pictured on the right is

A solution that has a pH reading of 6.8 is considered to be
A solution that has a pH reading of 7.8 is considered to be
A solution that has a pH reading of 0.8 is considered to be
A solution that has a pH reading of 13.8 is considered to be
A solution that has a pH reading of 7.0 is considered to be
A strong acid is added to a neutral solution. Describe the changes that occur in the pH of the solution.
A strong base is added to an acidic solution. Describe the changes that occur in the pH of the solution. Hint- an acid neutralizes a base.
Complete the table below. Look at the pH of each substance and decide if it is a weak or strong acid or base or may be neutral.
|
Substance
|
pH
|
Strong
or weak acid or base
|
| Blood |
8
|
|
| Toothpaste |
8
|
|
| Black coffee |
5
|
|
| Pure water |
7
|
|
| Stomach fluids |
1
|
|
| Tomatoe |
5
|
|
| Lemons |
2
|
|
| Laundry detergent |
11
|
|
| Oven cleaner |
13
|
|
| Sea water |
9
|
|
Continue (acids and bases around us)