Neutralisation reactions
A neutralisation reaction takes place when an acid and base react together. The reaction is given below.
acid + base => salt + water
You will need:
-1M Sodium hydroxide
-1M Hydrochloric acid
-evaporating crucible
-universal indicator
-measuring cylinder
-dropper
-glass stirring rod
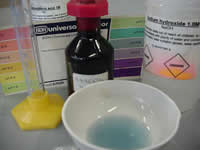


Click to see a 120kb video of the universal indicator changing colour when added to sodium hydroxide. What colour change indicates a strong base?
Click to see a 120kb video of hydrochloric acid been added to the sodium hydroxide. What colour change indicates an acid? Why does the solution turn green when the acid is mixed into the sodium hydroxide solution?

Describe how the pH of the stomach fluid changes as the antacid tablet or powder dissolves.
What are the products of the reaction between hydrochloric acid and the bases magnesium hydroxide and aluminium hydroxide?